Revista Fuentes: El Reventón Energético
Vol. 15 Nº 2 de 2017 - Jul/Dic - pp
31/47

EVIDENCES OF CDG FORMATION
AND POSSIBLE INTERPRETATIONS
OF CORE FLOOD STUDIES

Daniela Alzate López1*; Juan Manuel León2;
Fernando Cabrera3; Eduardo Manrique4 1 Universidad
Nacional de Colombia. Facultad de Minas. Calle 80 Carrera 65, Medellín,
Colombia.
2 Ecopetrol S.A. Carrera 7 Calle 32.
Bogotá D.C, Colombia.
3 Nalco Champion. Calle 100 Calle 19.
Bogotá D.C, Colombia.
4 MI3 Petroleum Engineering.
708 13th St., Golden, CO 80401, USA.
*E-mail:dalzatel@unal.edu.co
ABSTRACT
Colloidal Dispersion Gels (CDG’s) have been successfully
tested in several countries including Colombia. However, despite numerous
successful field results reported in the literature, laboratory-scale
experiments have generated controversy regarding the ability to inject CDG’s in
large volumes without reducing injectivity while also improving sweep
efficiency.
This paper summarizes the updates in microgel
technologies, especially the Linked-Polymer Solutions (LPS) that have been also
referred as CDG’s leading to misinterpretation of both systems. This brief
review will also present the main mechanisms proposed
for the formation of LPS in fluid:fluid studies and during its flow in porous
media. This study also presents for the first time
evidences of the possible mechanisms for the formation of CDG’s using a high
molecular weight (MW) partially hydrolyzed polyacrylamide (HPAM) and Aluminum
Citrate (Al(Cit)3) as a crosslinker using Dina Cretáceos Field,
Colombia, synthetic brine at room temperature (25°C).
The results generated during this study were used to re-interpret corefloods
injecting CDG in Berea and Tello Field, Colombia, core plugs at different
experimental conditions.
The main difference identified between LPS and CDG systems
is the viscosity behavior in the presence of Al(Cit)3. LPS reports a decrease in
viscosities while CDGs shows an increase in viscosities in the presence of
crosslinker. This difference is due to the use of different high MW HPAM
polymers. However, the crosslinking of the trivalent ion (Al3+) and
the negatively charged carboxylic groups of the polymer
of both microgels occurs through intra-and inter-molecular interactions leading
to different particle size or hydrodynamic diameter
distributions (HDD). The rate and type of HDD is dependent of polymer and
crosslinker concentration. These results were also compared with a CDG systems
using Chromium Acetate (Cr(Ac)3) as a crosslinker used in Loma Alta
Sur Field, Argentina. The crosslinkers used (Al3+ y Cr3+)
forming CDG suggests similar crosslinking mechanisms but
shows differences in HDD. However, the difference in the experimental
conditions of studies documented makes difficult developing a more detailed
comparison. Finally, the re-interpretation of CDG corefloods suggests that the
main operating mechanisms include viscosity effects,
adsorption, straining and log-jamming as proposed for LPS systems. However,
viscosity effects and the gradual blocking of pore channels (log-jamming) seem
to be more dominant in CDG than LPS systems. The results of this study will
contribute with the understanding of the CDG’s and also provides guidance to
improve the evaluation and research of the technology at lab scale.
Key words: CDG (Colloidal
dispersion Gels), Polyacrylamide, Hydrodynamic Diameter Distributions,
Microgels, Enhanced Oil Recovery (EOR).
Cita: Alzate, D., León, J.M., Cabrera, F. y Manrique, E. (2017). Evidences of CDG formation and possible interpretations of core flood studies. Revista Fuentes: El reventón energético, 15
(2), 31-47.
DOI: http://dx.doi.org/10.18273/revfue.v15n2-2017003
EVIDENCIAS DE LA FORMACIÓN DE CDG Y POSIBLES
INTERPRETACIONES DE PRUEBAS DE DESPLAZAMIENTO EN MUESTRAS DE NÚCLEOS
RESUMEN
La inyección de Geles de Dispersión Coloidal (CDG) ha sido evaluada
exitosamente en diferentes países incluyendo Colombia. Sin embargo y a pesar
del número de casos exitosos reportados en la literatura, estudios
experimentales han generado controversias respecto a la posibilidad de inyectar
altos volúmenes de CDG sin reducir la inyectividad y al mismo tiempo mejorar la
eficiencia de barrido en el yacimiento.
Este trabajo resume brevemente la actualidad de la tecnología de
microgeles, especialmente las Soluciones de Polímero Entrecruzadas (LPS) que
también ha sido referenciado como CDG generando confusiones en la
interpretación de ambos sistemas. Esta revisión también resume los mecanismos
propuestos para la formación de LPS basados en estudios de interacciones
fluido:fluido y durante su flujo en medios porosos. El presente estudio
documenta por primera vez las evidencias de los posibles mecanismos de la formación
de CDG utilizando una poliacrilamida parcialmente hidrolizada (HPAM) de alto
peso molecular y Citrato de Aluminio (Al(Cit)3) como entrecruzador
utilizando salmuera sintética del Campo Dina Cretáceos, Colombia, a condiciones
ambiente (25°C). Los resultados obtenidos en este estudio fueron utilizados
para re-interpretar pruebas de desplazamiento de inyección de CDG en muestras
de núcleo de Berea y del Campo Tello, Colombia, a diferentes condiciones
experimentales.
Se identifica que la principal diferencia entre el LPS y el CDG es el
comportamiento de la viscosidad en presencia de Al(Cit)3. El LPS
reporta una disminución de la viscosidad mientras que el CDG un aumento de la
misma al interactuar con este entrecruzador. Esta diferencia se basa
fundamentalmente en que estos sistemas se formulan con diferentes HPAM de alto
peso molecular. Sin embargo, se identifica que el entrecruzamiento del ion
trivalente (Al3+) y los grupos carboxílicos cargados negativamente
del polímero ocurre de manera similar para ambos sistemas a través de
interacciones intra- e inter-moleculares generando diferentes distribuciones de
tamaño de partículas o diámetros hidrodinámicos (DDH). La velocidad de reacción
y tipo de DDH resulta dependiente de las concentraciones de polímero y del
entrecruzador. Estos resultados se comparan con sistema CDG formulado con
Acetato de Cromo (Cr(Ac)3) como entrecruzador utilizado en el Campo
Loma Alta Sur, Argentina. Los entrecruzadores empleados para formar CDG (Al3+
y Cr3+) sugieren mecanismos de interacción similares pero generan
diferentes DDH. Sin embargo, las diferencias en las condiciones experimentales
de ambos estudios dificultan establecer comparaciones más detalladas. Finalmente,
la re-interpretación de pruebas de desplazamiento con CDG sugiere que los
principales mecanismos de efectos de viscosidad, adsorción, restricción y
divergencia del flujo resultan similares a los reportados para los sistemas
LPS. Sin embargo, se estima que los efectos de viscosidad y de bloqueo de
canales del medio poroso resultan más dominantes en los sistemas CDG respecto a
los LPS. Los resultados de este trabajo contribuyen con el mejor entendimiento
de los CDG y también sugiere guías para mejorar la evaluación e investigación de
la tecnología a escala de laboratorio.
Palabras Claves: CDG (Geles de Dispersión Coloidal), Poliacrilamida,
Distribuciones de Diámetros Hidrodinámicos, Microgeles, Recobro Mejorado de
Petróleo.
INTRODUCTION
porous media. CDG is one of several polymer microgel
technologies that have been proposed for in-depth Colloidal Dispersion Gels
(CDG’s) have been conformance and potentially as mobility
control method successfully tested in Argentina, China, USA, and most to
improve sweep efficiency in waterfloods. Abdulbaki, recently in Dina Cretáceos
Field, Colombia (Manrique, Huh, Sepehrnoori, Delshad & Varavei (2014)
recently et al., 2014). However, questions remain regarding reported a
literature review of different polymer the mechanisms operating during the CDG
flow in the microgel technologies (Table 1).
Table 1. Summary of polymer
microgel technologies discussed by Abdulbaki, et al., (2014).
|
Technology Gelation At
Surface Trigger In-Situ Particle Size Before Particle Size After
|
|
CDG
Preformed
CDG PPG
BrightWater
|
In-situ
Preformed
|
Polymer & crosslinker Microgel
Particle Gel
Microgel
|
Transition Pressure
Temperature
|
Swollen
microgel
Swollen
particle gel
Swollen microgel
|
nm to µm
µm to cm
0.1-1 µm
|
µm
20-200 larger
1-10 µm
|
|
pH-sensitive
|
|
|
pH
|
|
µm
|
Up to 1,000 larger
|
PPG = Preformed Particle Gels
Abdulbaki, et al. (2014) also provided a summary differentiating
polymer flooding, polymer in-situ gel flooding and polymer microgel flooding
technologies. However, this review does not differentiate Linked-
Polymer Solutions (LPS) and CDG technologies leading to
misinterpretations of both systems. Before describing the main differences of
LPS and CDG it is important provide a basic definition of these systems. LPS
and CDG are linked polymer solutions with properties like colloidal solutions.
Both systems are formed due to intra- and inter-molecular interactions of low
concentration of high MW HPAM and Al(Cit)3 as the crosslinker.
However, the viscosity and size distributions of LPS and CDG show important
differences that will be summarized in this section.
Aarra, et al. (2005) referred indistinctly in-depth
mobility control systems as LPS or CDG that are formed with low concentration
of partially hydrolyzed polyacrylamide polymer cross linked with aluminum citrate
(Al(Cit)3). However, detailed reviews of LPS studies confirmed that these systems are formed with SNF Flopaam 3630 (High MW HPAM) and Al(Cit)3 (Bjorvisk,
Hoiland, & Skauge, 2008) (Bolandtaba & Skauge, 2011) (Selle et al.,
2013) (Skauge, Hetland, Spildo, Skauge & Cipr, 2010) (Skauge, Djurhuus,
Hetland, Spildo & Skauge, 2011)generated by a crosslinking reaction between
aluminium and partially hydrolyzed Polyacrylamide (HPAM (Spildo, Skauge, Aarra
& Tweheyo, 2009) (Spildo, Skauge & Skauge,
2010). This represents the first difference with CDG systems that are formed with a different high MW HPAM commercially
available. Romero (2009) reported the effects of polymer type on the formation
of CDG in glass beads porous media.
The second difference found between these two systems is
that LPS viscosities are lower than the polymer solution at the same
concentration (Bjørsvik, Høiland & Skauge, 2008) while CDG viscosities are
higher in the presence of Al(Cit)3 compared to the polymer solution
at the same concentration (Alzate, 2016). This is also valid for CDG systems
prepared with Chromium Acetate (Diaz, et al., 2015).
Despite the differences between LPS and CDG systems, it is
important to remark that LPS studies documented in the literature reported new
insights for the re-interpretation of possible mechanisms of
CDG formation and its flow in porous media. A brief
summary of the main findings reported during research studies of LPS systems
are listed below:
LPS have properties similar to simple colloidal solution with particle size in the order of 20-150 nm (Aarra, et
al., 2005) (Bjorvisk et al., 2008) ( Bolandtaba & Skauge, 2011).
The initial crosslinking reaction is fast and takes several days to complete the reaction. The crosslinking of
trivalent ions (i.e. Al3+) and negatively
charged carboxylic groups of the polymer occurs through
intra- and inter-molecular interaction and its combination (Bjorvisk et al.,
2008) ( Spildo, et al., 2010).
LPS can propagate in porous media and increase oil recoveries (Spildo, et al., 2009) (Spildo, et al., 2010) (
Skauge, et al., 2010).
Main mechanisms reported during LPS injection include viscosity effects, adsorption, straining and log-jamming.
The microscopic diversion caused by gradual blocking of pore channels (caused
by the log-jamming mechanism) leads to diversion of
local flow mobilizing trapped oil. The effects of the
proposed mechanisms on the water relative permeability represent a balance
between the degree of blocking and the amount of produced oil ( Bolandtaba
& Skauge, 2011).
Based on the experienced gained evaluating LPS systems, this work is aimed at evaluating the CDG system injected in
Dina Cretáceos Field, Colombia. This study will use the
chemicals injected in the field and prepare the CDG in
synthetic brine to determine the effects of polymer concentration and
polymer:crosslinker ratio on the viscosity and size distribution over time. The
results of these experiments will be used to interpret
different coreflood studies developed to support the CDG injection in Dina Cretáceos and Tello Fields, Middle Magdalena
Valley, Colombia.
EXPERIMENTAL TESTS
Diaz, et al., (2015) reported for the first time the size distribution of CDG agglomerates formed during the interaction of
high molecular weight (MW) partially hydrolyzed polyacrylamide (HPAM) polymer
and Chromium Acetate (Cr(Ac)3). To further
expand the understanding of CDG systems, Alzate (2016)
developed a study evaluating the formation of CDG at lab scale using the same
HPAM and Aluminum Citrate (Al(Cit)3) as the
crosslinker (CLX). Specifically, this paper will summarize
the effects of HPAM and CLX concentration on the rheology and HDD of CDG
agglomerates formed over time.
Materials
To prepare the polymer and CDG solution synthetic water of
Dina Cretaceous Field was used. Dina synthetic water consists in NaHCO3
(0.99 g/L); NaCl (6.07 g/L); Na2SO4 (0.01 g/L); CaCl2*2H2O
(1.02 g/L); MgCl2*6H2O (0.52 g/L); BaCl2 (0.02
g/L); FeCl3*6H2O (0.03 g/L); Sr(NO3)2
(0.02 g/L); and KCl (0.10 g/L). The polymer was an HPAM (Nalco®EOR-370) with a
high MW (18-21 million Dalton) and a 30% hydrolysis. The Aluminum Citrate
(Nalco®EOR-677N) was chosen as the CLX. The CDG solutions were prepared from a
polymer stock solution of 2,000 ppm and a CLX stock solution of 1,000 ppm. The
polymer was dispersed in the synthetic water and was
mixed using a magnetic stirrer at low speed (125 RPM) for approximately 12
hours (overnight). The CLX was mixed in synthetic water to ensure its complete dissolution. It is important to mention that
all CDG systems prepared did not consider the use of
oxygen scavengers due to the low temperature (25°C) and short evaluation times
(≤ 7 days) of the experiments. Table 2
summarizes the CDG systems prepared using the stock solutions of HPAM and CLX.
Polymer concentrations and P:CLX ratio selected for this study are within the
range of the CDG system injected in Dina Cretáceos Field (Castro, et al.,
2013).
Table 2. CDG Samples.
|
Polymer
Polymer
–
Samples Concentration
Crosslinker
Ratio
(ppm)
|
Crosslinker
Concentration
( ppm )
|
|
1 Polymer
Base Line
|
400
|
|
|
2 40:1
|
400
|
10.00
|
|
3 40:1
|
600
|
15.00
|
|
4 40:1
|
200
|
5.00
|
|
5 20:1
|
400
|
20.00
|
|
6 60:1
|
400
|
6.67
|
Rheological and Hydrodynamic
Diameter Measurements
Rheological measurements were performed using a Fungilab – Alpha Series rotational
viscometer. All viscosity measurements were carried out a room temperature
25°C.
The Dynamic Light Scattering (DLS) method was used to
measure (NanoPlus – Zeta potential and Nano Particle
Analyzer) the hydrodynamic diameter of the polymer
and CDG systems. The
hydrodynamic size measured by the DLS can be defined as
“the size of a hypothetical hard sphere that diffuses
in the same way as the particle being measured” (Instruments, 2011). However,
the particles or macromolecules in solution are dynamic and non-spherical.
Therefore, the calculated diameter from the particle diffusion properties will
be an indicative of the apparent size of the dynamic particle.
The measurement principle of the DLS is based on the
Brownian motion that affects the particles dispersed in solution (Systems,
2012). The intensity and speed of this vibrational movement depend on the
temperature and the viscosity of the liquid. High temperature means more
movement. If the liquid contains a particle, that particle receives the
constant impacts of the molecules of the liquid. The vibrational velocity of
this particle also depends of its own size but the density and mass of the particle have no influence (Nelson, 1967). The Stokes-Einstein equation describes the dependence between the speed
of the movement and the size of the particles. Equation (1) presents the
Stokes-Einstein relation to calculate the particle size taking into account that the DLS method measures the diffusion coefficient and not the movement speed (Kaszuba, McKnight, Connah,
McNeil-Watson, & Nobbmann, 2008):
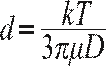
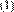
Where d is hydrodynamic diameter, K is the Boltzman constant, T is the temperature, µ is the viscosity of the solvent and D is the diffusion coefficient.
The DLS results are presented as diameter size
distribution respect to the differential intensity. The intensity distribution
is weighted according to the scattering intensity of each particle fraction or
particle family. This distribution may represent either a small amount of
agglomerated particles or a large particle.
As described in the equation (1), DLS measurement is
affected by the viscosity of the liquid. In the present study the viscosity
value measured at the lowest shear rate (10 rpm) was used as a reference for
measuring the hydrodynamic diameter of the CDG systems. It should be noted that
CDG or polymer solutions were not subjected to any degradation process before
the measurement of their hydrodynamic diameter. The viscosity as a function of the
shear rate and the hydrodynamic diameter of the CDG systems were evaluated at
0, 1, 3, 5, and 7 days after its preparation. All tests were performed at room
temperature.
RESULTS AND DISCUSSION
This section of the paper summarizes the results of CDG
viscosities and HDD (size) over time at different polymer concentrations and
polymer:crosslinker (P:CLX) ratios reported by Alzate (2016). The results of
this study will be used to re-interpret corefloods run
in Berea core plugs injecting CDG (using Al(Cit)3
as a crosslinker) and Dina Cretáceos Field fluids
(Castro, et al., 2013) (Manrique et al., 2014). Based
on the similarities of CDG viscosities and HDD using Al(Cit)3
(Alzate, 2016) and Cr(Ac)3 (Diaz, et al.,
2015), corefloods run in Tello core plugs injecting CDG
(using Cr(Ac)3 as a crosslinker) will be also discussed. The
re-interpretation of corefloods discussed in this paper
will also consider the evaluation of the proposed
mechanisms for the LPS systems (Bolandtaba & Skauge, 2011).
Viscosity and size distribution of CDG
system
Figure 1 shows the CDG viscosity as a function of polymer
concentration (200, 400 and 600 ppm) and time for a constant P:CLX ratio of
40:1. The black line represents the viscosity of the 400 ppm polymer solution
in absence of crosslinker (Al(Cit)3). It should be noted that CDG
viscosity increases with time and polymer concentration compared to the polymer
solution at 400 ppm. As can be seen in Figure 1, the CDG system prepared with
the HPAM solution of 200 ppm and
P:CLX of 40:1 reaches a viscosity of approximately 75cp at the seventh day of evaluation while the viscosity of the
HPAM solution at 400 ppm remains constant at about 11cp. This demonstrates that
CDG can achieve higher viscosities with less polymer concentration than polymer
solutions. The reported viscosity measurements have a precision of ±0.1cp with
a repeatability of 0.2%.
The viscosity behavior showed in Figure 1 is different
from that reported in the LPS systems evaluated by Bjørsvik, et al. (2008)
showing a decrease in viscosity over time. In addition, all
the LPS systems evaluated presented a viscosity reduction immediately after the
addition of the crosslinker agent.
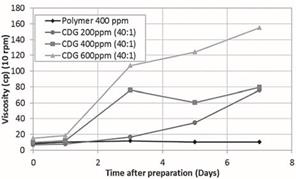
Figure 1. CDG
Viscosity as a function of polymer concentration and time at constant polymer:
crosslinker ratio (40:1).
Figure 2 shows the viscosity as a function of shear rate
and time for the CDG system of 400 ppm and P:CLX of 40:1. The viscosity of the
CDG systems after 1 day of its preparation is practically the same of the CDG
freshly prepared (Day 0). However, the viscosity significantly
increases after one day of interaction. CDG viscosity
continue increasing with time suggesting that the chemical interaction between
the HPAM and the crosslinker (Al(Cit)3) occurs at low reaction rates
and take place with time. Additionally, CDG systems evaluated
shows a Non-Newtonian fluid behavior, where its
viscosity depends on shear rate (Figure 2). This behavior is also similar for
the LPS systems evaluated by Bjørsvik, et al. (2008).
Figure 2. CDG
(400ppm & 40:1) viscosity as function of shear rate and time.
The HPAM solution of 400 ppm and the CDG system (400 ppm
and P:CLX ratio of 40:1) were selected to evaluate the HDD over time. The HDD
for the HPAM solution shows small variation over time. The HPAM solution only
develops a unimodal diameter distribution that is practically constant in the
time. The D50 is about 33.5 nm, and the particles size distribution is between
1.5 to 670 nm (Figure 3). On the contrary, the CDG systems evaluated at the
same polymer concentration shows a high variability of the HDD after 1 day of
interaction (Figure 4, Figure 5 and Figure 6). Figure 4 compares the HDD of the
HPAM solution of 400 ppm and the CDG system freshly prepared (at Day 0) at the
same polymer concentration and a P:CLX of 40:1. It can be noticed the
similarity between both distributions suggesting the slow interaction between
the polymer and the crosslinker to form the CDG agglomerates. This observation is
contrary with the fast initial crosslinking reaction reported for LPS systems.
However, both systems (CDG and LPS) requires several days to complete the
reaction (Bjorvisk, et al., 2008) (Spildo, et al., 2010).
However, after the first day of the preparation the
CDG solution the HDD shows a well-defined trimodal distribution indicating particles or agglomerates with average
diameters of 1.5 nm, 20 nm and 410 nm, respectively (Figure 5). Therefore, the
range of the size distribution increased from 2-380 nm (Day 0) to 1.5- 690 nm
(Day 1). The mean peak of 20 nm (dashed red line) can represent the HPAM
molecules which probably have not started its interaction with the crosslinker.
The peak observed at 410 nm can be interpreted as the formation of larger aggregates
or larger structures than the conventional HPAM. Finally, the peak registered
at 1.5 nm suggests the formation of small molecules (or colloids)
representative of intra-molecular interactions. These results are in agreement
with the crosslinking mechanisms of LPS systems that occurs through intra- and
inter-molecular interaction and its combinations as proposed by Bjorvisk, et
al. (2008) and Spildo, et al. (2010).
Figure 6 presents the HDD of the CDG system (400 ppm and
P: CLX of 40:1) from day 0 to day 7. It can be clearly noticed the strong
presence of the 3 welldefined peaks after 3 days of
interaction compared with diameter distributions
reported for days 0 and 1. The three peaks correspond to average values of 1.1
nm, 9 nm and 150 nm after 7 days of evaluation. It is remarkable two
characteristics of these trimodal distributions: the diameter size range of the
CDG system decreases over the time and the high intensity measured for the
particles with an average diameter of 1.1 nm. The differential intensity for
the particles in the range of 1.1 nm is around 30 to 40% of the cumulative
intensity of the sample. The particles in the range of 9 nm represent about 48%
and the cumulative intensity of the largest particles (peak @ 150 nm) is about
10 to 15%. This result suggests that the intramolecular interactions are
dominant in the formation of CDG and entail the formation of small agglomerates
or colloids. However, these results needs to be further investigated due to
possible uncertainties associated to the measurements of particles below the 1
nm range.
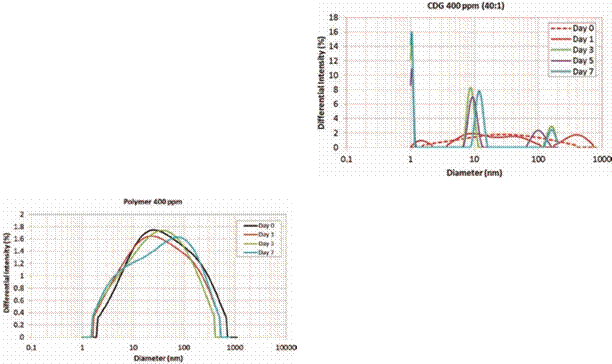
Figure 3.
Hydrodynamic Diameter as function of time – Polymer 400 ppm.
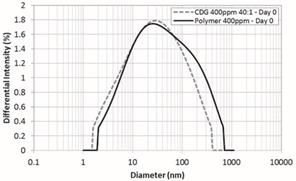
Figure 4. Hydrodynamic
Diameter at Day 0 – Polymer 400 ppm and CDG 400 ppm – 40:1
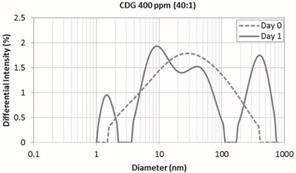
Figure 5. Hydrodynamic
Diameter at Day 0 and 1.
CDG 400 ppm – 40:1
Figure 6. Hydrodynamic
Diameter as function of time. CDG
400 ppm – 40:1
Figure 7, Figure 8, Figure 9 and Figure 10 shows the HDD
of CDG systems as function of polymer to crosslinker (P:CLX) ratio, polymer
concentration and time. The CDG system prepared with Dina Cretáceos Field
synthetic brine, HPAM solution of 400 ppm and P:CLX of 20:1 was selected for
evaluation. This system is the closest to the CDG injected in Berea corefloods that will be discussed in the next section of this paper. This CDG system shows three regions from the first day of measurements, where their modal peaks correspond to 1.4, 30 and 450 nm, respectively (Figure 7).
This behavior differs from the observed for the CDG of 400 ppm and P:CLX of
40:1 presented in
Figure 5 exhibiting a unimodal trend at Day 0 similar to the polymer solution in absence of Al(Cit)3. This
result suggests that an increase in the concentration of the crosslinker
accelerates the formation of agglomerates. As the time of reaction progress,
three regions are completely demarcated by their intensities for the days 3 and
5 (Figure 8). These peaks are identified around 1 nm, 8
to 11 nm and 100 to 160nm, respectively. However, for the day 7 a well-defined bimodal distribution is observed with peaks at 1 nm and 24 nm (solid
blue line). The particles in the range of 1 nm correspond to 56% of the sample
analyzed. Regardless the possible uncertainties associated to the measurements
of the hydrodynamic diameters in the range or below 1 nm, these results
demonstrates the effects of higher concentrations of crosslinker on the
formation of CDG agglomerates which seems to be dominated by intra-molecular
interactions. This can be also observed in the hydrodynamic diameter of CDG’s
prepared with a HPAM solution of 400 ppm at different P:CLX ratios as shown in
Figure 9. The sample with the highest concentration of crosslinker (20:1) shows
a less dispersed distribution at day 7 of evaluation (solid red line); where
unlike samples with P:CLX of 40:1 and 60:1 the largest particles disappear.
The effect of polymer concentration at a constant P:CLX
(40:1) on the HDD distribution is depicted in Figure 10. The polymer
concentration also affects the formation of the CDG particles with time. At
early stages of interaction (day 0) the increase in polymer concentration (from
400 to 600 ppm) and the same P:CLX (40:1) accelerates the intra- and
inter-molecular interactions. This behavior was also observed with the increase
of crosslinker concentration (Figure 7). As the reaction time progress in the
CDG system with the higher polymer concentration (600 ppm) shows a different
trend of the hydrodynamic diameter than the CDG’s with lower polymer
concentrations (200 and 400 ppm) at the same P:CLX (40:1). After seven days of
interaction, the agglomerates of larger size (>100 nm) disappears for the
CDG system (solid purple line) prepared with 600 ppm of polymer (Figure 10).
This trend was also observed with the increase of the crosslinker concentration
at the same polymer concentration of 400 ppm (Figure 9).
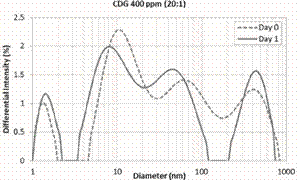
Figure 7. Hydrodynamic
Diameter at Day 0 and 1. CDG 400 ppm – 20:1
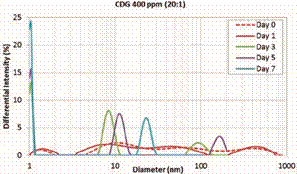
Figure 8. Hydrodynamic
Diameter as function of time. CDG 400 ppm – 20:1
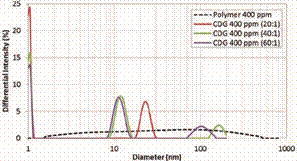
Figure 9. Hydrodynamic
Diameter as function of crosslinker concentration – Day 7.
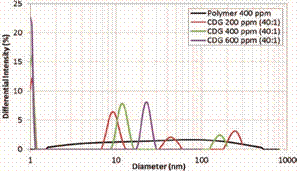
Figure 10. Hydrodynamic
Diameter as function of polymer concentration – Day 7.
Based on the results observed evaluating the HDD of CDG
systems prepared with HPAM polymer and
|
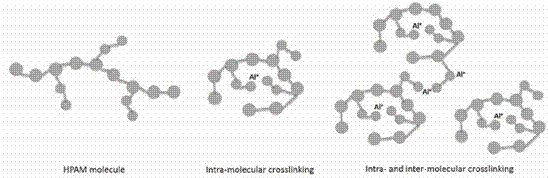
Figure 11. Schematic
representation of a HPAM molecule and intra- and inter-molecular crosslinking
with Aluminum (crosslinker).
|
Al(Cit)3 suggests that the initial reactions
occurs through intra- and inter-molecular interactions (Figure 11). This is
consistent with the crosslinking of Al3+and negatively charged carboxylic groups of the polymer of LPS systems proposed by (Bjorvisk, et al., 2008) (Spildo, et al., 2010). The
reaction rates of CDG systems also suggest to be influenced
by the polymer and crosslinker concentrations. The
higher the concentration the faster the formation of agglomerate structures of
different sizes. Additionally, as the reaction times increase the formation of
CDG systems seems to be dominated by intra-molecular forming structures (or
colloids) of smaller size. This effect becomes more evident as the polymer and
crosslinker concentration increases. It is worth to mention that the HDD
observed for different CDG systems (Figures 6, 8 and 9) shows that the
agglomerates formed are of smaller diameter than the polymer solution in
absence of crosslinker. This smaller HDD can be consider as a factor that may
limit the importance of the microscopic diversion (log-jamming) effects as
suggested in the literature (Bolandtaba & Skauge, 2011). However, the
microscopic diversions should be interpreted as a gradual blocking due to mechanical entrapment of agglomerates (mud filter cake type) as it will be presented later in this paper.