doi:10.18273/revion.v32n1-2019007
Saccharification of Orange Bagasse Pre-treated with Calcium
Hydroxide using an enzymatic blend Diluted Hydrochloric Acid
Sacarificación de bagazo de naranja pretratado con hidroxido de calcio
usando un cóctel enzimático y acido diluido
Sacarificação de bagaço de laranja pré-tratado com hidróxido de cálcio
usando um coquetel enzimático e ácido clorídrico diluído
Danielle
Pires-Nogueira1
Paula
Rubia Ferreira-Rosa2
Araceli
Aparecida Seolatto1
Carlos
Alberto Galeano-Suarez1
Fernanda
Ferreira-Freitas1*
1Department of Chemical
Engineering, Federal University of Goias, Campus Samambaia, Goiânia, Goiás,
Brazil.
2Department of Chemical
Engineering, Federal University of São Carlos, Rodovia Washington Luis, São
Carlos, São Paulo, Brasil.
*e-mail: fernanda_ferreira_freitas@ufg.br
Cita: Pires Nogueira D, Ferreira Rosa PR, Seolatto
AA, Galeano Suarez CA, Ferreira Freitas F. Saccharification of Orange Bagasse
Pre-treated with Calcium Hydroxide using an enzymatic blend Diluted
Hydrochloric Acid. rev.ion. 2019;32(1):75-85.
Creative Commons: https://creativecommons.org/licenses/by/4.0/
https://doi.org/10.18273/revion.v32n1-2019007
Fecha recepción: 1 de noviembre de 2018
Fecha aceptación: 28 de junio de 2019
Abstract
Enzymatic and dilute acid processes were applied to study
the orange bagasse hydrolysis. The moisture, ashes, lignin, cellulose, and
hemicellulose contents, of the orange peels, were quantified. The xylanase and
cellulase enzymes activities were quantified, as well as their optimum pH and
temperatures. The pre dried orange peel biomass was pre-treated with calcium
hydroxide, at preestablished conditions. The hydrolysis followed a central
composite factorial 2³ design. The cellulase activity was 28.05x10-6
FPU (Filter Paper Units)/m3, the optimum pH was 4.8 and the
temperature was 60°C. The results for xylanase were an activity of 199.58x10-3
U/Kg, pH 5.2, and temperature 50°C. The acid hydrolysis TRS (total reducing sugars)
values varied from (9.328±0.68 mg)*10-3 TRS per Kg of biomass to
(30.15±0.31)*10-3 mg TRS per Kg biomass, the most significant factor
was the temperature and the least the time. The enzymatic hydrolysis TRS values
varied from (77.33±3.82)*10-3 mg TRS per Kg biomass to
(99.66±0.62)*10-3 mg TRS per Kg biomass, the most significant factor
was the concentration of cellulase and the least the xylanase concentration.
Keywords: Central Composite Design; Biomass; Enzymes.
Resumen
La hidrólisis del bagazo de naranja fue
realizada por medio de un proceso enzimático con celulasas y un processo
químico con ácido diluido. Las cantidades de humedad, cenizas, lignina,
celulosa y hemicelulosa fueron cuantificadas. La actividad de las enzimas fue
determinada a temperatura y pH optimo. La biomasa fue pretratada con hidróxido
de cálcio. Los experimentos de hidrólisis fueron realizados utilizando un
diseño fatorial 2³ del tipo compuesto central. La actividad de la celulasa fue
de 28,05∙10-6 FPU (Filter Paper Units)/m3, con un pH
optimo de 4,8 y una temperatura de 60°C. Asimismo los resultados para la
actividad de xilanasas obtenidos fueron de 199,58∙10-3 U/ Kg, a pH
5,2, y temperatura 50°C. Los valores de azúcares reductores totales ART de la
hidrólisis ácida variaron de (9,328 ± 0,68)*103 Kg ART
/Kg de biomasa a (30,15±0,31)∙10-3 Kg ART/ Kg de
biomasa, presentando como factor mas significativo la temperatura y como menos
significativo, el tiempo. Para el caso de la hidrólisis enzimática los valores
de ART variaron de (77,33±3,82)∙10-3 Kg ART/ Kg de biomasa a
(99,66±0,62)∙10-3 kg ART / Kg de biomasa, siendo el fator
más significativo la concentración de celulasa y el menos significativo la
concentración de xilanasa.
Palabras clave: Diseño Compuesto Central; Biomasa; Enzimas.
Resumo
A hidrólise do bagaço de laranja foi
estudada por processos enzimáticos e ácido diluído. Os teores de umidade,
cinzas, lignina, celulose e hemicelulose foram quantificados. A atividade das
enzimas foi quantificada, bem como a temperatura e o pH ótimos. A biomassa foi
pré-tratada com hidróxido de cálcio. As hidrólises seguiram um planejamento
fatorial 2³ do tipo composto central. A atividade da celulase foi 28,05∙10-6
FPU (Filter Paper Units)/m3, o pH ótimo foi 4,8 e a temperatura foi 60°C.
Os resultados da xilanase foram atividade de 199,58∙10-3 U/Kg, pH
5,2, e temperatura 50°C. Os valores de açucares redutores totais (ART) da
hidrólise ácida variaram de (9,328 ± 0,68)∙10-3 Kg ART
por Kg de biomassa a (30,15±0,31)∙10-3 Kg ART por Kg biomassa, o
fator mais significativo foi a temperatura e o menos significativo, o tempo. Os
valores de ART da hidrólise enzimática variaram de (77,33±3,82)∙10-3
Kg ART por Kg biomassa a (99,66±0,62)∙10-3 kg ART por Kg biomassa, o
fator mais significativo foi a concentração de celulase e o menos significativo
a concentração de xilanase.
Palavras chave: Planejamento Composto Central; Biomassa; Enzimas.
Introduction
Biofuels are regarded as one of the most viable options for
reduction of CO2
emissions in the transport sector [1]. Human beings have been using biofuels
since pre-historical times. Wood and vegetable oil were the most common fuels
before the 19th
century, when they started been replaced by fossil fuel. Fossil fuels that
attend over 80% of the world’s energetic needs today. However, their reserves
are limited and are being depleted at alarming rates. Global warming, high oil
prices, and new investments have been leading to the search of renewable and
sustainable fuels. In spite of that, the so called 1st generation fuels can
get in the way of feeding people. But the lignocellulosic residues generated in
industrial processes can be applied to produce 2nd generation fuels that reduce solid residues
along with solving the energy problem. Besides being promising we still have a
long way to go before this new processes are economically viable [2-5].
One of the most researched biofuels has been ethanol; it can
be produced from multiple raw materials, such as, wood, agro-industrial
residues, municipal sewage, paper industry effluents, and different grasses.
The potential use of these materials is based on their large availability and
low cost [3, 6]. The biomass productions costs in Brazil are considered the
lowest in the world, with great possibilities of promising results [7].
Orange is vastly consumed all around the world, for its
flavor as well as its medicinal and nutritional properties.
Oranges are being processed as orange juice, therefore,
generating large amounts of waste that can be easily collected from a single
site, making it a promising biomass source. About 50% of processed orange is
estimated to be waste. Brazil is the world leader of orange production, with
35% of the world’s production. The orange bagasse contains approximately 16% of
hemicellulose, 28% of cellulose and 9% lignin, showing to be a viable
alternative to produce second generation ethanol. In order to obtain ethanol
from this residues it is necessary to hydrolyze the polysaccharides into
fermentable sugars, for posterior fermentation [8-10]. The presence of lignin
in lignocellulosic biomass makes it resistant to enzymatic attack to release
sugars. Pretreatment is an important step in bioethanol production in order to
achieve maximum saccharification efficiency with high fermentable sugar yields
[11]. Pretreatment is a fundamental step for refining biofuels and biobased
products from the lignocellulosic biomass as the bioconversion of cellulose
into fermentable monosaccharides [12]. Alkaline pretreatment, a kind of
pretreatment method, possesses some desirable features. It mainly removes
lignin and can be carried out at milder conditions than several other
pretreatments, like hydrothermal pretreatments, etc. [13]. Calcium hydroxide
(Ca(OH)2) is a
possible reagent to be used for alkaline pretreatment [14]. The most impacting
unitary operation is hydrolysis, and it can be either enzymatic or acid. The
acid hydrolysis can be either dilute or concentrated. For the dilute process
high temperatures are needed, and that can generate undesired compounds. Also,
with concentrated acids there can be difficulties in the process, such as
corrosion, and hydrolysate neutralizing costs. Depending on the conditions the
acid hydrolyses can remove the lignin and part of the hemicellulose opening
pores that give access to the cellulose and spare the pre-treatment phase.
Though, but in order to break the cellulose high temperatures or acid concentrations
are needed. While, the extreme conditions can generate inhibitory co-factors,
and degrade the sugars to other compounds reducing the hydrolysis efficiency.
Before fermenting it is necessary to neutralize the hydrolysate generating
salts that can’t be separated and can either act as supplement or inhibitor to
the yeast [3, 15, 16]. Lignocellulosic biomass from vegetable waste has a great
potential for use in the production of bioethanol. Although, due to its complex
structure, it requires pretreatment to improve the yield of reducing sugars in
the hydrolysate during enzymatic hydrolysis from cellulose and hemicellulose
[17]. For the enzymatic hydrolysis it is mandatory pre-treating the biomass to
make the cellulose accessible to the enzymes. Most studies about the
lignocellulosic ethanol production involve enzymes, besides that the
hydrolysate volumes are insufficient to the following fermentation processes. Even
with the reduction in the enzymes cost in the last 20 years, they still
represent around 50% of the second generation ethanol cost, that has a very superior
cost than first generation ethanol [2,15]. The aim with the present work was to
study the feasibility of obtaining total reducing sugars from orange peels. For
this, two types of hydrolysis, dilute acid and enzymatic, were evaluated. For
acid hydrolysis, it was studied the application of milder temperatures, to reduce
energy costs as well as the generation of inhibitory compounds. For enzymatic
hydrolysis, a combination of cellulase and xylanase was studied, in order to
increase the production of reducing sugars.
Materials and methods
Biomass
The orange peels were kindly donated by a juice processing
plant (Gyn Fruit in Goiânia GO Brazil). They were then cut into smaller pieces
and kilndried at 50°C until constant weight. The dried peels were crushed in
Wiley mill, and the fines, diameter under 0.3 m, were discarded for interfering
in the pre-treatment.
Biomass Characterization
The hemicellulose and cellulose content were
determined by the method described by Browning [11]. The ash and moisture
contents were determined by the Instituto Adolfo Lutz [19] methods. The lignin
was quantified using procedure preconized by IUPAC [20].
Pre-treatment
The pre-treatment was done using calcium hydroxide, biomass
and distilled water (1:4:20 w/w/v) at 60°C for 72000s and 150 rpm [9]. After the
pre-treatment the biomass was washed with 10 times the reaction volume with
distilled water and dried at 40°C until constant weight. After drying, the
biomass was stored in plastic vials at room temperature away from the light.
Enzymatic activities
To maximize the total reducing sugars (TRS) production two
types of enzymes were used, cellulase (Sigma-Aldrich) and xylanase (Granotec) both
from Trichoderma reesei. The choice to combine this enzymes was due to
the evidence of their synergic effect on the generation of sugars [14 -17].
The cellulase activity was determined using the paper filter
method, considering the effect of pH and temperature in the activity, the
results were expressed in FPU (Filter
Paper Units)/10-6. m3 [14, 25]. The activity of the xylanase was determined using
the procedure of the University of New South Wales (Kensington, Australia),
considering the effect of temperature and pH in the activity, and the results
expressed in U/Kg [19].
Hydrolyses central composite design
Factorial planning was used in order to evaluate the dilute
acid and enzymatic hydrolyses. The tests were planned using a factorial
planning of central composite type at two levels, with three variables (2³
assays), added of three replicas in the central point, to investigate a linear
model and also 6 experiments in the axial (α) points, bringing the total to 17
experiments. [27].
The dilute acid hydrolysis conditions for the codified and
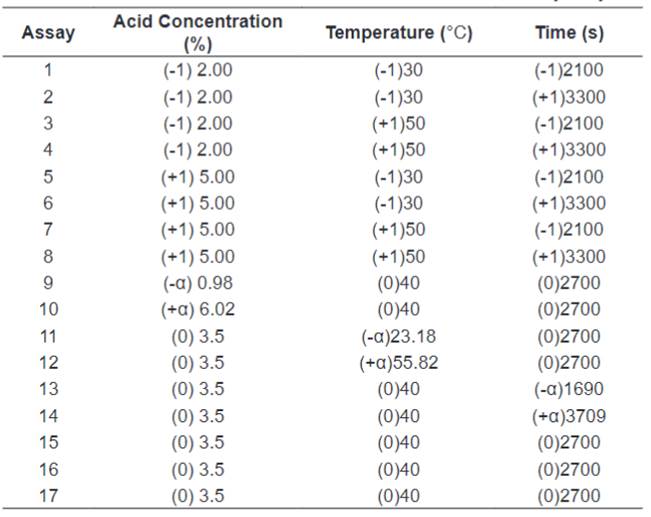
uncodified factors (acid concentration, time and temperature) are presented in Table 1. The factors are according Dussán et al. [15]. The
hydrolysis was done using 5.10-5 m3
of chloridric acid (HCl) solution in every concentration, and what was left
after the pre-treatment of 2∙10-3 Kg of biomass in 125∙10-6 m3 Erlenmeyer flasks.
Table 1.
Codified and uncodified factors for the dilute acid hydrolysis.
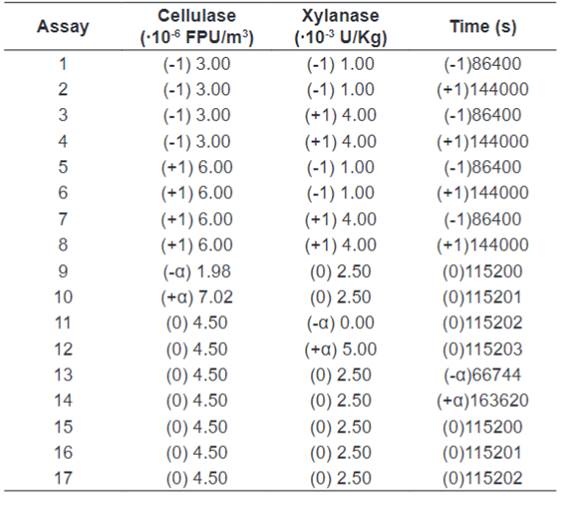
The enzymatic hydrolysis was carried out to evaluate the
impact of the enzymes concentration and time of hydrolysis. The solids
(cellulose and hemicellulose) were 1%. The buffer was sodium acetate, and the
rotation was 150 rpm. The temperature and pH were defined by the enzymatic activities
tests and constants have been kept for all experiments. Coded and non-codified
values are illustrated in the development matrix provided in Table
2. The codified alfa levels were 1.68179. In order to measure the amount of
total reducing sugars (TRS), after the hydrolyses, 1∙10-6m3 of the filtered was
measured and put in an assay tube, then 2∙10-6m3 of dinitrosalicylic acid reactant (DNS) were added. A
blank tube was done using 1∙10-6m3
of deionized water and 2∙10-6m3 of DNS. The tubes were
taken to a thermostatic bath at 95°C for 300 seconds followed by an ice bath to
stop the reaction. The volume was completed to 25∙10-6m3 and the absorbance was
read in spectrophotometer at the wave length of 540 nm [28].
Table 2.
Codified and uncodified factors for the enzymatic hydrolysis.
Results and discussion
Biomass Characterization
After being dried and milled the orange bagasse was sieved
and the particles under 0.295.103 m were discarded. The dry bagasse moisture content was
7.38%±0.03%, and the ashes content was 3.79%±0.04%. The cellulose contents were
22.9%±0.41%. The literature shows values that are similar to this [10, 29, 30].
The amount of hemicellulose was 3.39%±0.38%, value much
smaller than the researched references for orange bagasse [29-30]. The lignin
content was larger than the found for orange bagasse by other researchers
[29-30]. The composition of vegetal material may vary from different regions,
soil composition, and time of the year it was harvested [7].
Enzymatic Activities
The cellulase activity was 24.08∙10-6FPU/m3 the optimum pH was 4.8
and the optimum temperature was 60°C. For the xylanase the activity was
199.58∙10-3
U/Kg, the optimum pH was 5.2, but by a 3.4% difference to 4.8, and the optimum
temperature was 50°C. The pH of work was set at 4.8, and the temperature at
45°C.
Hydrolyses Central Composite Design
Statistical experimental design is one of the more efficient
and easier methods to arrange and interpret [31]. The results obtained in the
CCD showed that there was a good reproducibility between the central points,
statistically equal results, evidencing the quality of the repeatability of the
process. The highest response was obtained in experiment 12, performed at the
highest temperature used in the experiment (+ α). When comparing this
experiment with one of the central points there is a nearly 50% increase in the
value of the response. It is believed in the first instance that the
temperature variable has a strong influence on the release of ART after the
acid hydrolysis. It is observed a similarity of response between experiments 3
and 4, then it can be verified that when working with the lower level of acid
concentration, higher temperatures and increase of time between the lower and
upper levels the response does not change. With this, it can be inferred that
the best responses combine high temperatures and high acid concentrations, and
the time being less relevant.
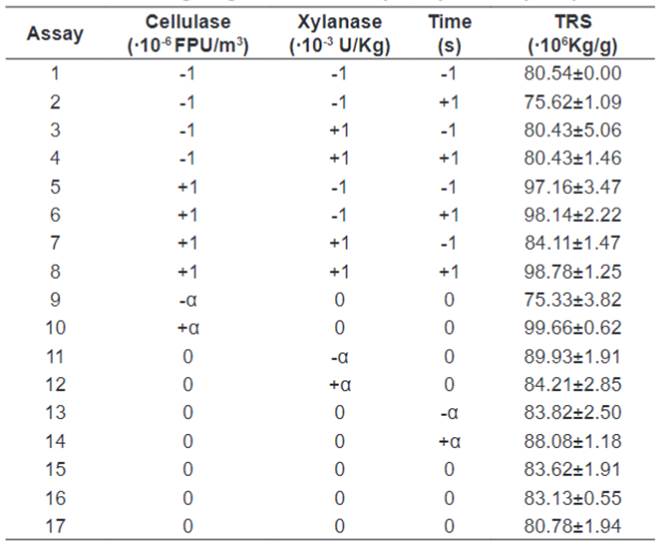
The average mass loss from the pre-treatment was 30.03%,
less than the reported by Silva et al. [9], 58.43%. For the dilute acid
hydrolysis the TRS levels varied from (9.32±0.68)∙10-3 Kg TRS per Kg of
biomass to (30.15±0.31)∙10-3
Kg TRS per Kg of biomass. All the results are presented in Table
3.
The multiple regressions of the variables were calculated
with the software Statistica ™ 7.0 and the significant terms (p≤0.1) are
presented in Table 4.
Table 3.
Amount of Total Reducing Sugars Obtained by Dilute Acid Hydrolysis of Orange
Bagasse.
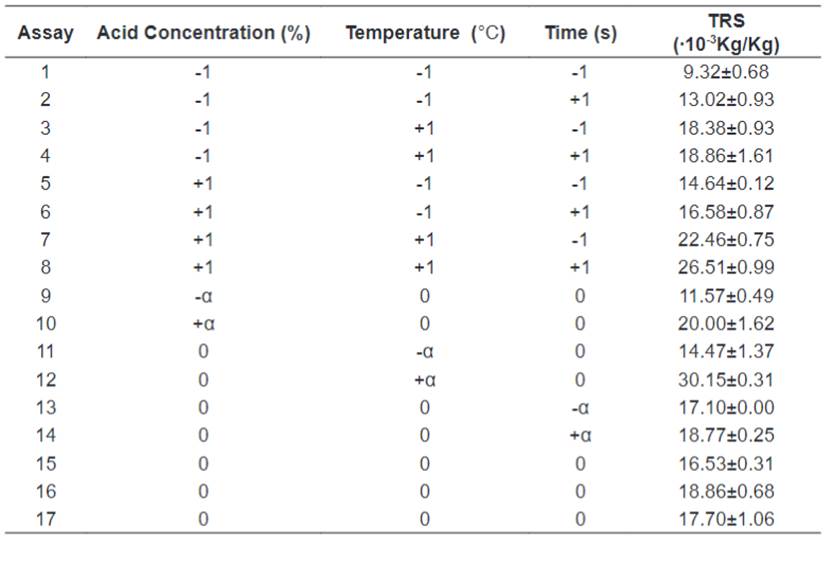
Table 4.
Significant Terms of the Dilute Acid Hydrolysis Results Multiple Regression.
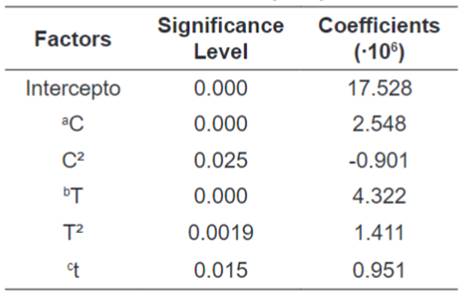
The reduced equation with a
determination coefficient R² of 96% was:
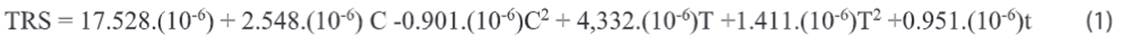
Where: TRS = Total Reducing
Sugars; C = HCl Concentration; T = Temperature and t = Time
Response Surface Methodology (RSM) is successfully used for
developing efficient bioprocesses. RSM is a statistical technique used for
experiment design, model building, estimating the effect of input variables and
predicting optimum conditions [32]. From the reduced equation the response
surfaces were constructed for each pair of independent variables in function of
the response variable TRS (Figure 1).
The Figure 1a showed that higher temperature levels led to
higher concentrations of ART combined with HCl concentrations at their lower
levels. When the time and the concentration of HCl are compared, (Fig. 1b) it
is observed that from intermediate concentrations of acid, a greater release of
ART occurs, while there is no substantial interference of the time, which can
be verified in the value of the coefficient of the time variable in Equation 1.
Similar analysis can be verified by evaluating the relation between time and
temperature, where the higher temperature levels, caused elevations in the ART levels,
while the time was not significant for the increase of sugar concentration
(Fig. 1c).
From the data obtained it is inferred that low temperatures
of dilute acid hydrolysis lead to a partial hydrolysis of the lignocellulosic
compounds, that is, a greater liberation of reducing sugars occurs at
temperatures higher than those studied in this work. However, when comparing
with the literature, it is seen that very high temperatures favor the
degradation of the formed sugars, reducing the yield of the process [15-16,33].
The Figure shows that the temperature was the most significant
factor for the generation of TRS, with time being the least significant. The
researched literature shows that with different biomasses and higher
temperatures (135°C – 175°C) the most impacting factor tends to be the acid
concentration [15]. The difference may be due to the fact that in this work the
optimum conditions were not found, and too high temperatures may degrade the
sugars, reducing the hydrolysis’ output [33].
The study of enzymatic hydrolysis after pretreatment with
calcium hydroxide was performed with the enzymes cellulase and xylanase at pH
4.8 and 150 rpm at 1% solids. Through statistical planning the concentration of
TRS achieved in the enzymatic hydrolysis varied from around 75.10-6 Kg TRS per 10-3 Kg of biomass to
almost 100.10-6
Kg/ 10-3Kg
(Table 5). It is possible to observe that the results of total reducing sugars
released by the enzymatic hydrolysis were about 3 times higher than those of
the diluted acid hydrolysis. This result may indicate that, although the
optimal acid hydrolysis conditions have not been found, enzymatic hydrolysis is
more efficient for the generation of sugars from the orange bagasse.
It can be observed from Table 5 that the highest concentrations
of ART occurred in experiments 5, 6, 8 and 10, corresponding to the higher
levels of cellulase concentration (+1 and +α) and variable levels of xylanase
concentration and temperature. The lowest values of ART were in experiments 2
and 9 that have in common the lower levels of cellulase, -1 and -α
respectively. It can be observed that the concentration of xylanase practically
did not interfere in the release of ART, which can be observed when comparing
experiments 1 and 3 that with equal levels of cellulase and time and different
levels of xylanase show practically the same concentration of ART.
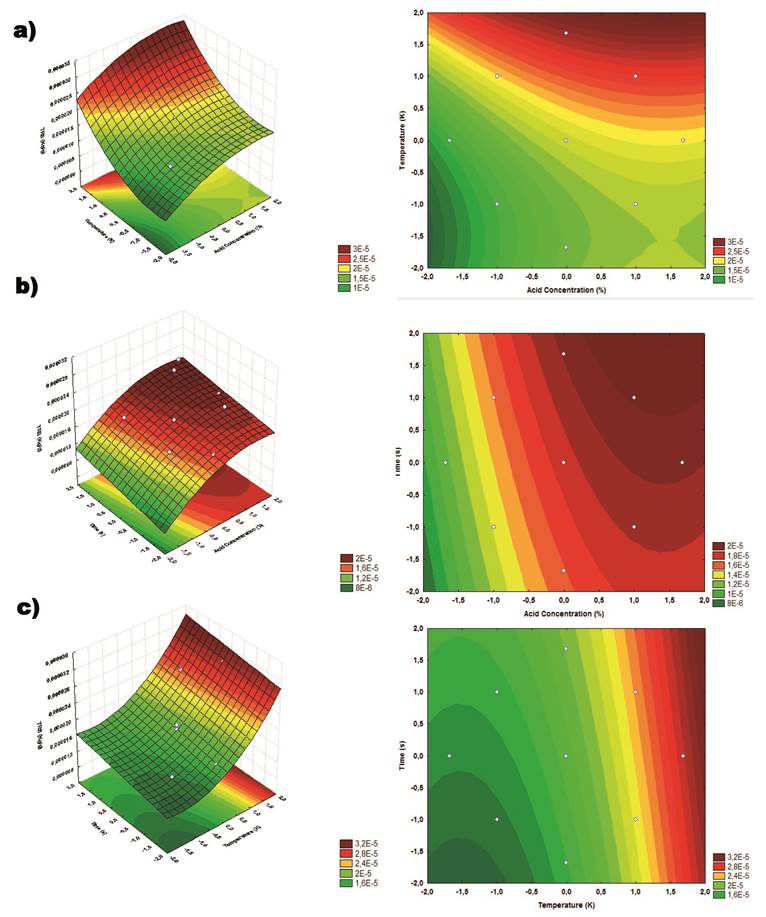
Figure
1.Response Surfaces and Contour Plots of the TRS (Kg.g-1): (a) HCl
Concentration (C) vs. Temperature (T);
(b) HCl Concentration
(C) vs. Time (t); (c) Temperature (T) vs. Time (t)
Table 5. Amount
of Total Reducing Sugars Obtained by Enzymatic Hydrolysis of Orange Bagasse.
The multiple
regressions of the variables was all the terms were significant (p≤0.1) and are
calculated with the software Statistica ™ 7.0, presented in Table
6.
Table 6.
Terms of the Dilute Acid Hydrolysis Results Multiple Regression.
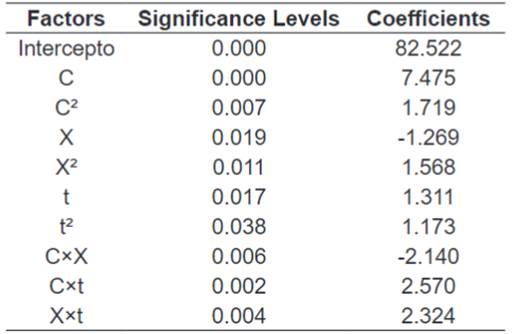
The whole equation,
with a determination coefficient R² of 98.3% was:
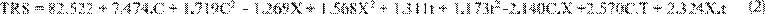
Where: TRS = Total Reducing Sugars, C = Cellulase
Concentration, X = Xylanase Concentration t = Time.
With the whole equation the response surfaces were
constructed for each pair of independent variables in function of the response
variable TRS (Figure 2).
The results demonstrate the relationship between enzyme
concentrations in the release of total reducing sugars, the highest values were
observed in the highest concentrations of cellulase (7.02∙10- 6 FPU/m3) and lower
concentrations of xylanase (0.00∙10-3 U/Kg), with the cellulase concentration being
more significant. When comparing the effect of time and cellulase concentration
on the concentration of total reducing sugars, it was observed that the higher
levels of the two variables (40 h and 7.02 ∙10-6 FPU/m3) led to higher concentrations of reducing
sugars. In the time relationship with the xylanase concentration, two maximum
regions were found for the liberation of total reducing sugars, one in the
combination of the lowest levels (0.0∙10-3 U/Kg and 18.54 h) and the other in the
combination of the highest levels (5.00∙10-3 U/Kg and 45.45 h)
The most significant factor for the generation of TRS was
the concentration of cellulose, the other factors, besides being significant,
were very much less impacting. Many authors have reported synergistic effects
on the hydrolysis of sugars by the combination of cellulase and xylanase, for
different substrates [21-24]. However, the results obtained in this work
indicate the opposite, the best results were at the highest levels of cellulase
and lowest of xylanase. When comparing the time and cellulase effect on the
generation of sugars the best results were at the combination of the highest levels
of both, which is in accordance with the literature [21-22].
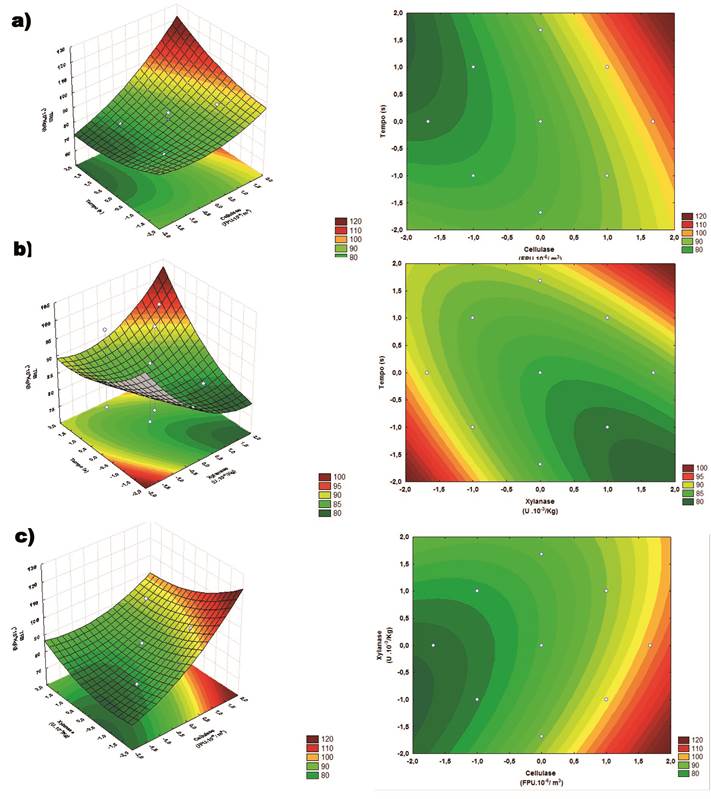
Figure
2. Response Surfaces and Contour Plots of the TRS (mg g-1): (a) Cellulase
Concentration (C) vs. Time (t); (b) Xylanase Concentration (X) vs.
Time (t); (c) Cellulase Concentration (C) vs. Xylanase Concentration
(X).
Conclusions
The temperatures and acid concentrations were insufficient
to totally hydrolyze the sugars in the biomass, requiring further
investigations with higher levels of both. The enzymatic hydrolysis yielded
higher amounts of total reducing sugars (TRS) than the acid hydrolysis,
indicating it could be more advantageous for further studies. The xylanase concentration
did not impact the enzymatic hydrolysis of the orange bagasse. The studied
conditions of time and cellulase concentration were not enough to totally
hydrolyze the substrate, also requiring further studies at different
conditions.
Acknowledgements
The authors gratefully acknowledge the financial support
provided by CNPQ and FAPESP São Paulo Research Foundation.
References
1. Ohm YK, Hwang KR, Kim C, Kim JR, Lee JS. Recent
developments and key barriers to advanced biofuels: A short review. Bioresour.
Technol. 2018;257:320-33.
2. Acker RV, Leplé JC, Aerts D, Storm V, Goeminne, G,
Ivens B, et al. Improved saccharification and ethanol yield from field grown
transgenic poplar deficient in cinnamoyl-CoA reductase. PNAS.
2014;111(2):845-50.
3. Canizo JR, Cortes-Callejas ML,
Davila-Gomez
FJ, Heredia-Olea E, Perez-Carrilloa E,
SernaSaldívar SO. Release of potentially fermentable sugars during
dilute acid treatments of bermuda grass NK37 (Cynodon dactylon) for
second-generation ethanol production. J. Chem. Technol. Biotechnol. 2014;89:1941-47.
4. Guo M, Song W, Buhain J. Bioenergy and biofuels:
History, status and perspectives. Renew. Sust. Energ. Rev. 2015;42:712-25.
5. Kosinkova J, Doshi A, Maire J, Ristovski Z, Brown R,
Rainey TJ. Measuring the regional availability of biomass for biofuels and the
potential for microalgae. Renew. Sust. Energ. Rev. 2015;49:1271-85.
6. Cotana F, Cavalaglio G, Gelosia M, Nicolini A, Coccia
V, Petrozzi A. Production of bioethanol in a second generation prototype from
pinewood chips. Energ. Proc. 2014;45:42-51.
7. Silva CEF, Gois GNSB, da Silva LMO, Almeida RMRG, Abud
AKS. Citric waste saccharification under different chemical treatments. Acta
Sci. Technol. 2015;37(4):387-95.
8. Kumar CSC, Mythily R, Chandraju S. Extraction of
carbohydrate from sweet orange peels (Citrus sinensis L.) and their
identification via LC/MS & thin layer chromatographic analysis. Biosci.
Biotech. Res. Asia. 2011;8(2):709-15.
9. Silva KA, Godoy PHM, Cardoso J, Mendes TPP, Seolatto
AA, Freitas FF. Study of orange bagasse digestibility by chemical
pretreatments. Chem. Eng. Trans. 2013;35:1045-50.
10. Souza CB, Jonathan M, Saad SMI, Schols HA, Venema K.
Characterization and in vitro digestibility of by-products from Brazilian food
industry: Cassava bagasse, orange bagasse and passion fruit peel. Bioact.
Carbohydr. Dietary Fibre. 2018;16:90-9.
11. Pandiyan, K, Singh, A, Saxena, A. K, Nain, L.
Technological interventions for utilization of crop residues and weedy biomass
fors second generation bio-ethanol production. Renew. Energy. 2019;132:723-41.
12. Akhtar N, Gupta K, Goyal D, Goyal A. Recent advances in
pretreatment technologies for efficient hydrolysis of lignocellulosic biomass.
Environ. Prog.Sustain. Energy. 2016;35:489511.
13. Kim JS, Lee YY, Kim TH. A review on alkaline pretreatment
technology for bioconversion of lignocellulosic biomass. Bioresour. Technol.
2016;199:42-8.
14. Chang M, Li D, Wang W, Chen D, Zhang Y, Hu H, Ye X.
Comparison of sodium hydroxide and calcium hydroxide pretreatments on the
enzymatic hydrolysis and lignin recovery of sugarcane bagasse. Bioresour.
Technol. 2017;244:1055–58.
15. Dussán KJ, Silva DDV, Moraes EJC, Arruda PV, Felipe
MGA. Dilute-acid hydrolysis of cellulose to glucose sugarcane bagasse. Chem.
Eng. Trans. 2014;38:433-38.
16. Lenihan P, Orozco A, O’Neill E, Ahmad MNM, Rooney DW,
Walker GM. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J.
2010;156:395-403.
17. Robak K, Balcerek M. Review of Second Generation
Bioethanol Production from Residual Biomass. Food Tech. & Biotech. 2018;56(2):174-87.
18. Browning BL. Methods of wood chemistry. USA, New York:
Wiley & sons; 1967.
19. Padilha P, Medeiro M, Duarte V,
Figueiredo E, Abreu P, Zenebon C. Métodos Químicos
e Físicos para Análise de Alimentos.
Digital. Brazil, São Paulo: Normas Analíticas do Instituto Adolfo Lutz; 2008.
20. Rabelo Cândida S. Avaliação e
otimização de pré-tratamentos e hidrólise enzimática do bagaço de
cana-de-açúcar para a produção de etanol de segunda geração. (Masters
Dissertation) Campinas, Brazil: Universidade Estadual de Campinas; 2010.
21. Bura R, Chandra R, Saddler J. Influence of xylan on the
enzymatic hydrolysis of steampretreated corn stover and hybrid poplar.
Biotechnol. Progr. 2009;.25(2):315-22.
22. Lin L, Yan R, Liu Y, Jiang W. In-depth investigation of
enzymatic hydrolysis of biomass wastes based on three major components:
Cellulose, hemicellulose and lignin. Bioresour. Technol. 2010;101:8217-23.
23. Selig MJ, Knoshaug EP, Adney WS, Himmel ME, Decker SR.
Synergistic enhancement of cellobiohydrolase performanceon pretreated corn
stover by addition of xylanase and esterase activities. Bioresour. Technol.
2008;99:4997-5005.
24. Xin D, Sun Z, Viikari L, Zhang J. Role of
hemicellulases in production of fermentable sugars from corn stover. Industr.
Crops Prod. 2015;74:209-17.
25. Adney B, Baker J. Measurement of Cellulase Activities.
Laboratory Analytical Procedure. Golden, USA: National Renewable energy
Laboratory. 2008.
26. Ghose TK, Bisaria VS. Measurement of hemicellulase
activities part 1: Xylanases. Great Britain. Pure Appl.
Chem.1987;59(12):173952.
27. Box GEP, Hunter JS. Multi-factor experimental designs
for exploring response surfaces. Ann. Math. Stat. 1987;28(1):195-241.
28. Miller GL. Use of dinitrosalicylic acid reagent for
determination of reducing sugar. Anal. Chem. 1959;31(3):426-28.
29. Retore M, Silva LP, Toledo GSP, Araújo IG. Efeito da fibra de coprodutos agroindustriais e sua avaliação
nutricional para coelhos. Arq. Bras. Med. Vet. Zootec. 2010;62(5):1232-40.
30. Rivas B, Torrado A, Torre P, Converti
A, Domínguez JM. Submerged citric acid fermentation on orange peel
autohydrolysate. J. Agric. Food. Chem. 2008;56:2380-7.
31. Sathendra E R, Baskar G, Praveenkumar R, Gnansounou E.
Bioethanol production from palm wood using Trichoderma reesei and Kluveromyces
marxianus. Bioresource Tech. 2019;271:345-52.
32. Baskar G, Selvakumari IAE, Aiswarya, R. Biodiesel
production from castor oil using heterogeneous Ni doped ZnO nanocatalyst.
Bioresour. Technol. 2018;250:793-8.
33. Awan TAJ A. Orange Bagasse as Biomass for 2G-Ethanol
Production. (Ph.D. Thesis) Campinas, Brazil: Universidade Estadual de Campinas;
2013.