Projeto e implementação de um sistema de pirólise catalítica de poliestireno expandido
Publicado 2024-12-09
Palavras-chave
- Poliestireno expandido,
- Pirólise catalítica,
- Estireno,
- Catalisador FCC
Como Citar
Copyright (c) 2024 Julieth García Sánchez, Lizeth Alejandra Arenas Aguilar, Lizeth Dayana Cendales Sánchez, Víctor Gabriel Baldovino Medrano

Este trabalho está licenciado sob uma licença Creative Commons Attribution-NoDerivatives 4.0 International License.
Resumo
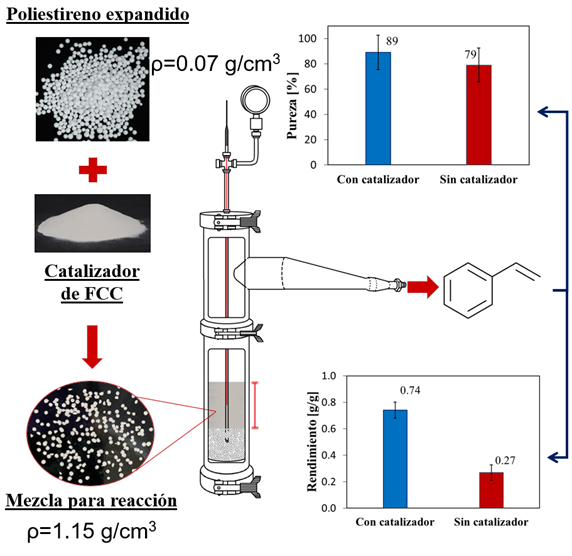
Esta pesquisa teve como objetivo projetar e colocar em operação um reator semicontinuo de aço inoxidável 316 para o aproveitamento de poliestireno expandido na obtenção de estireno por pirólise catalítica. Um reator de 1335 cm3 foi construído, composto por um recipiente tubular com saída lateral de vapores, um forno de aquecimento, um condensador tipo serpentina e um rotâmetro para a alimentação de um gás de arrastre (N2). Um catalisador industrial de craqueamento catalítico fluidizado foi utilizado para o processo. A metodologia desenvolvida para a realização dos testes catalíticos destaca-se por: (i) a mistura do catalisador com o poliestireno por meio de moagem mecânica; (ii) a determinação dos perfis de temperatura do reator e a comparação do comportamento do catalisador com o observado em sua ausência. A eficiência do processo foi avaliada em termos de rendimento de estireno e pureza do produto líquido. Finalmente, as melhores condições de operação foram determinadas por meio de um delineamento fatorial 32, utilizando como variáveis de entrada a temperatura (360 °C, 430 °C e 500 °C) e a altura do leito (4, 6 e 8 cm). Foi determinado que as variáveis afetam a pureza do líquido e o rendimento de estireno de forma semelhante, mas que o efeito gerado depende da interação entre as duas variáveis estudadas. As melhores condições de operação do reator obtidas a partir do delineamento experimental foram 430 °C e 4 cm de leito, obtendo-se um rendimento de 0,74 g de estireno/g de poliestireno expandido e 89 % de pureza.
Downloads
Referências
- Herberz T, Barlow CY, Finkbeiner M. Sustainability Assessment of a Single-Use Plastics Ban. Sustainability. 2020;12(9):3746. https://doi.org/10.3390/su12093746
- Dey A, Dhumal CV, Sengupta P, Kumar A, Pramanik NK, Alam T. Challenges and possible solutions to mitigate the problems of single-use plastics used for packaging food items: a review. J Food Sci Technol. 2021;58:3251–3269. https://doi.org/10.1007/s13197-020-04885-6
- Laville S, Taylor M. El mundo compra un millón de botellas de plástico por minuto que acaban en vertederos o en el mar. elDiario.es. https://www.eldiario.es/internacional/theguardian/compra-botellas-plastico-mayoria-vertederos_1_3309129.html (June 2017).
- Greenpeace. Situación actual de los plásticos en Colombia y su impacto en el medio ambiente. http://greenpeace.co/pdf/2019/gp_informe_plasticos_colombia_02.pdf (November 2019).
- Rodríguez DK. Colombia produce 1,4 millones de toneladas de plástico al año. Portafolio 2 June 2022. https://www.portafolio.co/economia/colombia-produce-1-4-millones-de-toneladas-de-plastico-al-ano-566367 (2 June 2022).
- Ministerio de Ambiente y Desarrollo Sostenible. Plan Nacional para la Gestión Sostenible de los plásticos de un solo uso. Bogotá, https://www.minambiente.gov.co/wp-content/uploads/2022/02/plan-nacional-para-la-gestion-sostenible-de-plasticos-un-solo-uso-minambiente.pdf (June 2021)
- Rodríguez H, Montilla T. Icopor, asesino silencioso de la vida humana (Tesis de Pregrado). Santiago de Cali, Colombia: Universidad Libre, Seccional Cali; 2021.
- García N. Evaluación del impacto ambiental de la aplicación de un plan de gestión posconsumo de poliestireno expandido (EPS) utilizado en el envase de alimentos en Colombia (Tesis de Maestría). Bogotá, Colombia: Universidad EAN; 2019.
- Barrera Castro GP. Caracterización de las propiedades mecánicas y térmicas de muestras de EPS pos consumo, utilizadas en la industria de alimentos y sometidas a un proceso de recuperación (Tesis de Maestría). Bogotá, Colombia: Universidad Nacional de Colombia; 2016.
- Chen W, Hao H, Hughes D, Shi Y, Cui J, Li ZX. Static and dynamic mechanical properties of expanded polystyrene. Materials & Design. 2015;69:170–180. https://doi.org/10.1016/j.matdes.2014.12.024
- Arthuz L, Pérez W. Alternativas De Bajo Impacto Ambiental Para El Reciclaje Del Poliestireno Expandido a Nivel Mundial. Informador Técnico. 2019;83(2):209–219. https://doi.org/10.23850/22565035.1638
- Arandes J, Bilbao J, López-Valerio D. Reciclado de residuos plásticos. Revista Iberoamericana de Polímeros. 2004;5:28–45.
- Contreras F. Estudio de la pirólisis catalítica de polietileno en un reactor semi-Batch (Tesis de Maestría). Santiago de Chile, Chile: Universidad de Chile; 2014.
- Mo Y, Zhao L, Chen C-L, Tan GYA, Wang J-Y. Comparative pyrolysis upcycling of polystyrene waste: thermodynamics, kinetics, and product evolution profile. J Therm Anal Calorim. 2013;111:781–788. https://doi.org/10.1007/s10973-012-2464-6
- Jaime Sepúlveda RM. Síntesis de resinas catalíticas para la transformación del glicerol en fase acuosa (Tesis de Pregrado). Bucaramanga, Colombia: Universidad Industrial de Santander; 2020.
- Zambrano A. Reciclaje químico de plástico mediante pirólisis catalítica usando un catalizador regenerado (Tesis de Pregrado). Riobamba, Ecuador: Escuela superior politécnica de Chimborazo; 2022.
- Miller RR, Newhook R, Poole A. Styrene production, use, and human exposure. Crit Rev Toxicol. 1994;24:S1–S10. https://doi.org/10.3109/10408449409020137
- Çelikgöğüs Ç, Karaduman A. Thermal-catalytic Pyrolysis of Polystyrene Waste Foams in a Semi-batch Reactor. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2015;37:2507–2513. https://doi.org/10.1080/15567036.2011.626492
- Williams PT, Bagri R. Hydrocarbon gases and oils from the recycling of polystyrene waste by catalytic pyrolysis. Int J Energy Res. 2004;28:31–44. https://doi.org/10.1002/er.949
- Fuentes C, Colman Lerner J, Vázquez P, Sambeth J. Analysis of the emission of PAH in the thermal and catalytic pyrolysis of polystyrene. Catalysis Today. 2021;372:175–182. https://doi.org/10.1016/j.cattod.2020.11.030
- Dong D, Tasaka S, Inagaki N. Thermal degradation of monodisperse polystyrene in bean oil. Polymer Degradation and Stability. 2001;72:345–351. https://doi.org/10.1016/S0141-3910(01)00031-3
- Inayat A, Fasolini A, Basile F, Fridrichova D, Lestinsky P. Chemical recycling of waste polystyrene by thermo-catalytic pyrolysis: A description for different feedstocks, catalysts and operation modes. Polymer Degradation and Stability. 2022;201:109981.
- Rawlence DJ, Gosling K. FCC catalyst performance evaluation. Applied Catalysis. 1988;43(2):213–237. https://doi.org/10.1016/S0166-9834(00)82729-3
- Palos R, Rodríguez E, Gutiérrez A, Bilbao J, Arandes JM. Cracking of plastic pyrolysis oil over FCC equilibrium catalysts to produce fuels: Kinetic modeling. Fuel. 2022;316:123341. https://doi.org/10.1016/j.fuel.2022.123341
- Lee C-G, Cho Y-J, Song P-S, Kang Y, Kim J-S, Choi M-J. Effects of temperature distribution on the catalytic pyrolysis of polystyrene waste in a swirling fluidized-bed reactor. Catalysis Today. 2003;79–80:453–464. https://doi.org/10.1016/S0920-5861(03)00060-9
- Wang J, Ma Y, Li S, Yue C. Catalytic pyrolysis of polystyrene in different reactors: Effects of operating conditions on distribution and composition of products. JAAP. 2024;177:106366. https://doi.org/10.1016/j.jaap.2024.106366
- Imani Moqadam S, Mirdrikvand M, Kharaghani A, Roozbehani B, Shishehsaz MR. Polystyrene pyrolysis using silica-alumina catalyst in fluidized bed reactor. Clean Techn Environ Policy. 2015;17:1847–1860. https://doi.org/10.1007/s10098-015-0899-8
- Budsaereechai S, Hunt AJ, Ngernyen Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv .2019;9:5844–5857. https://doi.org/10.1039/C8RA10058F
- Inayat A, Klemencova K, Grycova B, Sokolava B, Lestinsky P. Thermo-catalytic pyrolysis of polystyrene in batch and semi-batch reactors: A comparative study. Waste Manag Res. 2021;39:260–269. https://doi.org/10.1177/0734242X20936746
- Hussain Z, Imtiaz M, Naz MY, Khan KM, AbdElSalam NM, Ibrahim KA. Thermal and clinker‐catalyzed pyrolyses of polystyrene waste using the Portland cement solid‐base catalyst. Asia-Pacific J Chem Eng. 2021;16:e2556. https://doi.org/10.1002/apj.2556
- Adnan, Shah J, Jan MR. Effect of polyethylene terephthalate on the catalytic pyrolysis of polystyrene: Investigation of the liquid products. J. Taiwan Inst. Chem. Eng. 2015;51:96–102. https://doi.org/10.1016/j.jtice.2015.01.015
- Shah J, Jan MR, Adnan. Tertiary recycling of waste polystyrene using magnesium impregnated catalysts into valuable products. JAAP. 2015;114:163–171. https://doi.org/10.1016/j.jaap.2015.05.009
- Gonzalez-Aguilar AM, Pérez-García V, Riesco-Ávila JM. A Thermo-Catalytic Pyrolysis of Polystyrene Waste Review: A Systematic, Statistical, and Bibliometric Approach. Polymers. 2023;15(6):1582. https://doi.org/10.3390/polym15061582
- Taipe Andagua JG. Obtención de combustibles a partir de residuos de polipropileno reciclado, mediante pirólisis catalítica (Tesis de Pregrado). Latacunga; Ecuador: Universidad de las Fuerzas Armadas ESPE. Extensión; 2021
- Sergeev OA, Shashkov AG, Umanskii AS. Thermophysical properties of quartz glass. Journal of Engineering Physics. 1982;43:1375–1383. https://doi.org/10.1007/BF00824797
- Pérez Bravo G, Contreras Larios JL, Rodríguez González JF, Estrada Pérez JE. Obtención de estireno a partir de residuos de poliestireno expandido mediante pirolisis catalítica. Revista tediq. 2021:7(7):201–205.
- Kannan P, Biernacki JJ, Visco DP. A review of physical and kinetic models of thermal degradation of expanded polystyrene foam and their application to the lost foam casting process. JAAP. 2007;78(1):162–171. https://doi.org/10.1016/j.jaap.2006.06.005
- Baldovino-Medrano VG. Diseño de experimentos: una introducción pragmática. Bucaramanga (Colombia): Ediciones UIS, 2023.
- Montgomery DC. Design and analysis of experiments. 5th ed. New York: John Wiley; 2001.
- Tukey JW. Comparing Individual Means in the Analysis of Variance. Biometrics. 1949;5(2):99-114. https://doi.org/10.2307/3001913
- Medina Molano NS, Roa Pinto JS. Efecto de la inhibición del carbazol sobre el hidrocraqueo de fenantreno (Tesis de Pregrado). Bucaramanga, Colombia: Universidad Industrial de Santander; 2017.
- Huang J, Li X, Meng H, Tong H, Cai X, Liu J. Studies on pyrolysis mechanisms of syndiotactic polystyrene using DFT method. Chemical Physics Letters. 2020;747:137334. https://doi.org/10.1016/j.cplett.2020.137334
- García-Sánchez JT, Mora-Vergara ID, Molina-Velasco DR, Henao-Martínez JA, Baldovino-Medrano VG. Key factors during the milling stage of the seed assisted and solvent-free synthesis of MFI and catalytic behavior in the alkylation of phenol with tert-butyl alcohol. ChemCatChem. 2021;13(16):3713–3730. https://doi.org/10.1002/cctc.202100479
- Mercado DF, Ballesteros-Rueda LM, Lizarazo-Gómez CC, Núñez-Rodríguez BE, Arenas-Calderón E, Baldovino-Medrano VG. Synthesis and use of functionalized SiO2 nanoparticles for formulating heavy oil macroemulsions. Chemical Engineering Science. 2022;252:117531. https://doi.org/10.1016/j.ces.2022.117531
- Hernández-Maya MS, Espinosa-Lobo CB, Cabanzo-Hernández R, Mejía-Ospino E, Baldovino-Medrano VG. Effects of pH and vanadium concentration during the impregnation of Na-SiO2 supported catalysts for the oxidation of propane. Molecular Catalysis. 2022;520:112158. https://doi.org/10.1016/j.mcat.2022.112158
- Loftus GR. On interpretation of interactions. Mem Cogn. 1978;6:312–319. https://doi.org/10.3758/BF03197461
- Wagenmakers E-J, Krypotos A-M, Criss AH, Iverson G. On the interpretation of removable interactions: A survey of the field 33 years after Loftus. Mem Cogn. 2012;40:145–160. https://doi.org/10.3758/s13421-011-0158-0
- Rosnow RL, Rosenthal R. “Some Things You Learn Aren’t So”: Cohen’s Paradox, Asch’s Paradigm, and the Interpretation of Interaction. Psychol Sci. 1995;6(1):3–9. https://doi.org/10.1111/j.1467-9280.1995.tb00297.x
- Sachanen AN, O’Kelly AA. High-Temperature Alkylation of Aromatic Hydrocarbons. Ind Eng Chem. 1941;33(12):1540–1544.
- Lu C, Xiao H, Chen X. Simple pyrolysis of polystyrene into valuable chemicals. e-Polymers. 2021;21:428–432. https://doi.org/10.1515/epoly-2021-0037
- Adnan, Shah J, Jan MR. Thermo-catalytic pyrolysis of polystyrene in the presence of zinc bulk catalysts. J. Taiwan Inst. Chem. Eng. 2014;45:2494–2500. https://doi.org/10.1016/j.jtice.2014.05.011

