Effect of ionic composition in water: oil interactions in adjusted brine chemistry waterflooding: preliminary results
Published 2018-12-18
Keywords
- Low Salinity Waterflooding (LSW), Adjusted Brine Composition Waterflooding (ABCW), Improved Oil Recovery (IOR), Enhanced Oil Recovery (EOR), Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS), Electrospray Ionization (ESI).
How to Cite
Copyright (c) 2018 Revista Fuentes

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
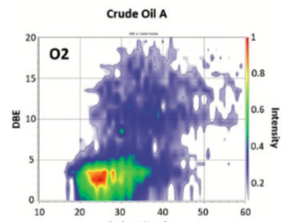
Low salinity or adjusted brine composition waterflooding (LSW or ABCW) is considered a promising improved/enhanced oil recovery (IOR/EOR) method. Despite the large number of studies documented in the literature, there are contradictory results and a lack of consensus regarding the mechanisms that operate in this recovery process. The proposed fluid:rock and fluid:fluid mechanisms are still under discussion and investigation. However, the impact of oil geochemistry and its importance on the fluid:fluid interactions that can occur with brines during LSW or ABCW have been overlooked and studied in a lesser extent. The scope of the present study is to preliminary evaluate crude oil:brine interactions to validate the influence of its compositions. These interactions were evaluated at static conditions for a week and reservoir temperature (60°C) using two oil samples from different Colombian basins and brine solutions of different composition at a constant ionic strength (I = 0.086). Specifically, this investigation evaluated the effect of the type of cation (Na+ and Ca2+) and anion (Cl- and SO4=) on crude oil:brine interactions. The results of these experiments were compared with tests using distilled water (DW). Although a basic characterization of brines (i.e. pH, alkalinity and ionic composition) and oil (oil viscosity) was performed, the main objective of this study is the analysis of water-soluble organic compounds (WSOC) using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS). The results demonstrate that water:oil interactions are dependent on brine and crude oil compositions. The main changes observed in the aqueous phase were the increase in inorganic components (desalting effects) and organic compounds soluble in water. Only the system crude oil A and NaCl (5,000 ppm) showed the formation of a micro dispersion. Negative electrospray ionization (ESI (-)) FT-ICR MS data shows that WSOC’s identified in DW and Na2SO4 after the interaction with crude oil A belongs to similar classes but there is marked selectivity of species solubilized with different brines. The relative abundance of classes Ox, OxS and NOx (x > 2) decreases while Ox, OxS and NOx (x ≤ 2) increase their solubility in the presence of Na2SO4 compared to DW. The analysis of O2 and O3S classes using double bond equivalence (DBE) vs. carbon number (CN) contour plots shows that the isoabundance of water-soluble species are within the range of DBE £ 10 and CN £ 20 regardless the brine used in the experiments. Finally, the method of solvent extraction in silica columns used in this investigation for the analysis of WSOC using FT-ICR MS represents a powerful and new approach to study LSW and ABCW.
Downloads
References
Alvarado, V., García-Olvera, G., Manrique, E. (2015). Considerations of Adjusted Brine Chemistry for Waterflooding in Offshore Environments. Offshore Technology Conf., Rio de Janeiro, Brazil, Oct. 27-29.
Ayirala, S. C., Li, Z., Saleh, S. H., Xu, Z., Yousef, A. A. (2018). Effects of Salinity and Individual Water Ions on Crude Oil-Water Interface Physicochemical Interactions at Elevated Temperature (SPE-190387). SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, March 26-28.
Bae, E. J., Yeo, I. J., Jeong, B., Shin, Y., Shin, K. H., Kim, S. (2011). Study of Double Bond Equivalents and the Numbers of Carbon and Oxygen Atom Distribution of Dissolved Organic Matter with Negative-Mode FT-ICR MS. Analytical Chemistry, 83, 4193-4199.
Bernard, G. G. (1967). Effect of Floodwater Salinity on Recovery of Oil from Cores Containing Clays (SPE-1725). SPE/AIME Annual California Regional Meeting. pp. 1-8.
Chakravarty, K. H., Fosbøl, P. L., Thomsen, K. (2015). Brine Crude Oil Interactions at the Oil-Water Interface (SPE-174685). SPE EOR Conference, Kuala Lumpur, Malaysia, August 11-13.
Collins, I. R., Couves, J. W., Hodges, M., McBride, E. K., Pedersen, C. S., Salino, P. A., Webb, K. J., Wicking, C., Zeng, H. (2018). Effect of Low Salinity Waterflooding on the Chemistry of the Produced Crude Oil (SPE-190191). SPE IOR Conference, Tulsa, OK, USA, April 14-18.
De Oliveira, M., Ribero, M. A., Weitzel, M. T., Carneiro, D., Brandão G. P., Ribeiro de Castro, E. V., Fonsecada, F. L., Oliveira, W., De Queiroz Ferreira, R. (2015). Evaluation and determination of chloride in crude oil based on the counterions Na, Ca, Mg, Sr and Fe, quantified via ICP-OES in the crude oil aqueous extract. Fuel, V. 154, Aug. 15, pp. 181- 187. https://doi.org/10.1016/j.fuel.2015.03.079
Fisher J. B. (1987). Distribution and occurrence of aliphatic acid anions in deep subsurface waters. Geochimica Cosmochimica Acta, 51, 2459 -2468.
Fjelde, I. F., Polanska, A. P., Taghiyev, Asen, S. M. A. (2013). Low Salinity Water Flooding: Retention of Polar Oil Components in Sandstone Reservoirs (Paper A24). 17th European Symp. on Improved Oil Recovery, St. Petersburg, Russia, April, 16-18.
Forero, J., Duque, J., Díaz, J., Nuñez, A., Guarin, F., Carvajal, F. (2001). New Contact System in Crude Oil desalting Process. Ciencia Tecnología y Futuro (CT&F), V. 2, No. 2, Jan./Dec.
García-Olvera, G., Reilly, T. M., Lehmann, T. E., Alvarado, V. (2016). Effects of asphaltenes
and organic acids on crude oil-brine interfacial visco-elasticity and oil recovery in low-salinity waterflooding. Fuel, 185: 151-163.
Giordano, T. H., Kharaka, Y. H. (1994). Organic ligand distribution and speciation in Sedimentary basin brines, diagenetic fluids and related ore solutions. Geological Society, London, Special Publications, V. 78, pp: 175- 202. DOI: 10.1144/GSL.SP.1994.078.01.14.
Gonsior, M., Valle, J., Schmitt-Kopplin, P., Hertkorn, N., Bastviken, D., Luek, J., Harir, M., Bastos, W., Enrich-Prast, A. (2016). Chemodiversity of dissolved organic matter in the Amazon Basin. Biogeosciences, 13, 4279-4290.
Helgeson, H. C., Knox, A. M., Owens, C. E., Shock, E. L. (1993). Petroleum, oil field waters, and authigenic mineral assemblages: Are they in metastable equilibrium in hydrocarbon reservoirs? Geochimica Cosmochimica Acta, 57, 3295 - 3339.
Kaasa, B., Østvold, T. (1997). Alkalinity in Oil Field Waters. What Alkalinity is and How it is Measured (SPE-37277). SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, Feb. 16-21.
Kellerman, A. M., Dittmar, T., Kothawala, D. N., Tranvik, L. J. (2014). Chemodiversity of dissolved organic matter in lakes driven by climate and hydrology. Nature Comm. 5:3804. doi: 10.1038/ncomms4804.
Khatib, Z. I., Salanitro, J. P. (1997). Reservoir Souring: Analysis of Surveys and Experience in Sour Waterfloods (SPE-38795). SPE Annual Technical Conference and Exhibition, San Antonio, TX, Oct. 5-8.
Kilybay, A., Ghosh, B., Thomas, N. C. (2017). A Review on the Progress of Ion - Engineered Water Flooding. J. Pet. Eng., vol. 2017, No. 13 February, pp. 1-9.
Kujawinski, E.B., Kido Soule, M.C., Valentine, D.L., Boysen, A.K., Longnecker, K., Redmond, M.C. (2001). Fate of Dispersants associated with the Deepwater Horizon oil spill. Environ Science Technology; 45(4), 1298-306.
Lafargue, E., Barker, C. (1988). Effect of Water Washing on Crude Oil Compositions. The American Association of Petroleum Geologist Bulletin, V. 72, No. 3, March. pp: 263 - 276.
Larter, S. R., Aplin, A. C. (1995). Reservoir Geochemistry: methods, applications and opportunities. Geological Society, London, Special Publications, v. 86; p5-32. DOI: 10.1144/GSL.SP.1995.086.01.02.
MacGowan, D. B., Surdam, R. C. (1988). Difunctional carboxylic acids anions in oilfield waters. Organic Geochemistry, 12 (3), 245 - 289.
Mahzari, P., Sohrabi, M. (2015). Impact of Micro- Dispersion on Effectiveness of Low Salinity Waterflooding (Paper Tu B09). 18th European Symposium on Improved Oil Recovery, Dresden, Germany, April 14-16.
Manning, F. S., Thompson, R. E. (1995). Oilfield Processing Volume two: Crude Oil. PennWell Publishing Company. ISBN 0-87814-354-8.
Martin, C. A. G., Páez, E. G. M. (2017). Efeito da salinidade na tensão interfacial do sistema óleo/ agua em condições isobáricas e incremento gradual da temperatura. Fuentes: El reventón energético, 15(2), 117-124. https://revistas. uis.edu.co/index.php/revistafuentes/article/ view/7689.
Muller, H., Hajji, A.A., Koseoglu, O. R. (2007). Proceedings Extended Abstracts. Chemindex, Manama, Bahrain, March.
Rojas-Ruiz, F. A., Gómez-Escudero, A., Pachón- Contreras, Villar-García, A., Orrego-Ruiz, J. A. (2016). Detailed Characterization of Petroleum
Sulfonates by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy & Fuels, 30, 2714-2720.
Skauge, A. (2013). Low Salinity Flooding – A critical Review (Paper A11). 17th European Symposium on Improved Oil Recovery, St. Petersburg, Russia, April, 16-18.
Sleighter, R. L., Hatcher, P. G. (2007). The application of electrospray ionization coupled to ultrahigh resolution mass spectrometry for the molecular characterization of natural organic matter. Journal of Mass Spectrometry, 42: 559-574.
Stanford, L. A., Kim, S., Klein, G. C., Smith, D. F., Rodgers, R. P., Marshall, A. G. (2007) Environmental Science & Technology, Vol. 41, No. 8: 2696 – 2702.
Surdam, R. C., MacGowan, D. B. (1987). Oilfield waters and sandstone diagenesis. Applied Geochemistry, 2, 613 - 619.
Turner, A. (2003). Salting out of chemicals in estuaries: Implications for contaminant partitioning and modelling. Science of the Total Environment, 314: 599-612.
Wang, L., Kan, A. T., Zhang, Z., Yan, F., Liu, Y., Dai, Z., Tomson, M. B. (2014). Field Methods for Determination of Bicarbonate Alkalinity (SPE-169758). SPE International Oilfield Scale Conference and Exhibition, Aberdeen, UK, May 14-15.
Wang, X., Alvarado, V. (2012). Effects of the Aqueous-Phase Salinity on Water-in-Crude Oil Emulsion Stability. Journal of Dispersion Science and Technology, 33: 165-170. DOI: 10.1080/01932691.2010.548689.