Polymers for application in high temperature and high salinity reservoirs – critical review of properties and aspects to consider for laboratory screening
Published 2018-12-18
Keywords
- Harsh Reservoirs, Polymer Flooding, Laboratory Screening, Enhanced Oil Recovery (EOR) High Temperature and High Salinity Reservoirs.
How to Cite
Copyright (c) 2018 Revista Fuentes

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
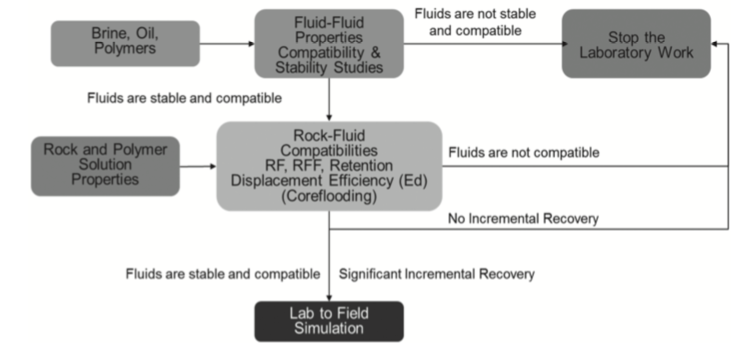
A significant amount of oil resides in deep reservoirs characterized by relatively high temperature and high salinity. In such reservoirs, most available chemicals fluids for EOR have limited applicability. Even though recent effort has been dedicated to the development of high temperature polymers, there is no clear understanding of what would work best in those harsh environments. In addition, the oil and gas community is also evaluating potential applications of chemical EOR to offshore assets where similar conditions are often found. Field applications in harsh reservoirs have shown limited success in the use of polymers for improved oil recovery. Detail analysis reveals that screening of the fluids was done under ‘model’ laboratory conditions, using non-reservoir core samples and non-representative fluids. These facts have motivated research and development work towards understanding the type of polymers that may be suitable for use in high temperature and high salinity reservoirs and to determine the type of tests to use to assess their performance in a field application for use as lab screening criteria. In this paper, we provide a critical review of the available polymers for application in high temperature and high salinity reservoirs and summarize best practices for their laboratory screening though a recommended workflow.
Downloads
References
Akbari, S., Mahmood, S. M., Tan, I. M., Ghaedi, H., & Ling, O. L. (2017). Assessment of Polyacrylamide Based Co-Polymers Enhanced by Functional Group Modifications with Regards to Salinity and Hardness. Polymer, 9 (7), 647-662. DOI:10.3390/polym9120647.
Alcázar-Vara, L. A., Zamudio-Rivera, L. S., Buenrostro-González, E., Hernández- Altamirano, R., Mena-Cervantes, V. Y., & Ramírez-Perez, J. F. (2015). Multifunctional Properties of Zwitterionic Liquids. Application in Enhanced Oil Recovery and Asphaltene Aggregation Phenomena. Ind. Eng. Chem. Res., 54 (11), 2868–2878. DOI: 10.1021/ ie504837h.
Alexis, D., Varadarajan, D., Kim, D. H, Winslow, G., & Malik, T. (2016). Evaluation of Innovative Associative Polymers for Low Concentration Polymer Flooding. (SPE 179696). SPE Improved Oil Recovery Conference held in Tulsa, Oklahoma, USA. DOI: 10.2118/179696-MS.
American Petroleum Institute. (1998). Recommended Practices for Core Analysis, API RP40. Washington, DC, USA: American Petroleum Institute.
American Petroleum Institute. (1990). Recommended Practices for Evaluation of Polymers Used in Enhanced Oil Recovery Operations. API RP 63. Washington DC, USA: American Petroleum Institute.
Araujo, Y. C., Araujo, M., & Molinaris, J. (2018). Best Practices for Laboratory Evaluation of Immiscible WAG. (SPE 190303). SPE Improved Oil Recovery Symposium held in Tulsa, Oklahoma, USA. DOI: 10.2118/190303-MS.
Borthakur, A., Rahman, M., Sarmah, A., & Subrahmanyam, B. (1995). Partially Hydrolyzed Polyacrylamide for Enhanced Oil Recovery. Res. Ind. 2, 90-94.
Caram, Y., Bautista, F., Puig, J., & Manero, O. (2006). On the Rheological Modelling of Associative Polymers. Rheologica Acta, 46, 45–57.
Chen, C. S. H., & Sheppard, E. W. (1980). Conformation and Shear Stability of Xanthan Gum in Solution. Polym. Eng. Sci., 20 (7), 512-516.
Dai, C., Xu, Z., Wu, Y., Zou, C., Wu, X., Wang, T., Guo, X., & Zhao, M. (2017). Design and Study of a Novel Thermal-Resistant and Shear-Stable Amphoteric Polyacrylamide in High-Salinity Solution. Polymers, 9, 296-310.
DiPaola-Baranyi, G., & Guillet, J. E. (1978). Estimation of Polymer Solubility Parameters by Gas Chromatography. Macromolecules, 11 (1), 228-235.
Doe, P. H., Moradi-Araghi, A., Shaw, J. E., & Stahl, G. (1987). Development and Evaluation of EOR Polymers Suitable for Hostile Environments - Part 1: Copolymers of Vinylpyrrolidone and Acrylamide. SPE Reservoir Engineering, 2(4), 461-467.
Domenico, P. A., & Schwartz, F. W. (1990). Physical and Chemical Hydrogeology. First Edition, New York, USA: John Wiley & Sons.
Dupuis, G., Antignard, S., Giovannetti, B., & Gaillard, N. (2017). A New Thermally Stable Synthetic Polymer for Harsh Conditions of Middle East Reservoirs. Part I. Thermal Stability and Injection in Carbonate Cores. (SPE 188479). Abu Dhabi International Petroleum Exhibition & Conference held in Abu Dhabi, UAE. DOI:10.2118/188479-MS.
Escudier, M. P., Clement-Evans, J., & Poole, R.J. (2005). Freezing as a Storage Process for Aqueous Polymer Solutions. Applied Rheology, 15(2), 90-97.
Fan, Y., Boulif, N., & Picchioni, F. (2018). Thermo- Responsive Starch-g-(PAM-co-PNIPAM):Controlled Synthesis and Effect of Molecular Components on Solution Rheology. Polymers, 10, 92-103.
Gaillard, N., Giovannetti, B., Favero, C., Caritey, J. P., Dupuis, G., & Zaitoun, A. (2014). New Water Soluble NVP Acrylamide Terpolymers for Use in EOR in Harsh Conditions. (SPE 169108). SPE Improved Oil Recovery Symposium Held in Tulsa, Oklahoma, USA. DOI: 10.2118/169108-MS.
Garcı́a-Ochoa, F., Santos, V. E., Casas, J. A., & Gómez, E. (2000). Xanthan Gum: Production, Recovery, and Properties. Biotechnology Advances, 18 (7), 549-579. DOI: 10.1016/ S0734-9750(00)00050-1.
Gibbons, M. K., & Örmeci, B. (2013). Quantification of Polymer Concentration in Water Using UV- VIS Spectroscopy. Journal of Water Supply: Research and Technology, 62 (4), 205-213.
Hashmet, M. R., AlSumaiti, A. M., Qaiser, Y., & AlAmeri, W. S. (2017). Laboratory Investigation and Simulation Modeling of Polymer Flooding in High-Temperature, High-Salinity Carbonate Reservoirs. Energy Fuels, 31, 13454−13465. DOI: 10.1021/acs. energyfuels.7b02704.
Hinkley, R., & Brown, G. (2017). Polymer Enhanced Oil Recovery – Industry Lessons Learned. Oil & Gas Authority. Retrieved from www.ogauthority.co.uk.
Holzwarth, G., Soni, L., & Schulz, D. N. (1986). Molecular Weight Distribution of Water- Soluble Polymers: A New Absolute Method. Macromolecules, 19 (2), 422–426. DOI: 10.1021/ma00156a032.
Hou, C. T., Barnabe, N., & Greaney, K. (1986). Purification and Properties of a Novel Xanthan Depolymerase from a Salt-Tolerant Bacterial Culture, HD1. Appl. & Environ. Microbiology, 52 (1), 37-44.
Izunobi, I. U., & Higginbotham, C. L. (2011). Polymer Molecular Weight Analysis by 1H NMR Spectroscopy. J. Chem. Educ., 88 (8), 1098–1104.
Jensen, T, Kadhum, M., Kozlowicz, B., Summer, E. S., Malsam, J., Muhammed F., & Ravikiran, R. (2018). Chemical EOR Under Harsh Conditions: Scleroglucan as a Viable Commercial Solution. (SPE 190216). SPE Improved Oil Recovery Symposium Held in Tulsa, Oklahoma, USA. DOI: 10.2118/190216-MS.
Jian, S., Huang, T. Y., Wang, K. M., Tang, B. Z., & Yu, Q. (2016). Determination Method of the Solubility Parameter of Polymer Based on AIE. Molecules, 22, 54-59.
Kang, J., Sowers, T. D., Duckworth, O. W., Amoozegar, A., Heitman, J. L., & McLaughlin, R. A. (2014). Turbidimetric Determination of Anionic Polyacrylamide in Low Carbon Soil Extracts. Journal of Environ. Quality, 42 (6), 1902-1907.
Kierulf, C., & Sutherland, I. W. (1988). Thermal Stability of Xanthan Preparations. Carbohyd. Polym., 9 (3), 185-194.
Lara-Ceniceros, A., Rivera-Vallejo, C., & Jimenez-Regalado, E. (2007). Synthesis, Characterization and Rheological Properties of Three Different Associative Polymers Obtained by Micellar Polymerization. Polymer Bulletin, 58, 425–433.
Laschewsky, A. (2014). Structures and Synthesis of Zwitterionic Polymers. Polymers, 6, 1544-1601.
Leela, J. K., & Sharma, G. (2000). Studies on Xanthan Production from Xanthomonas Campestris. Bioprocess Eng., 23 (6), 687-689.
Levitt, D. B. (2009). The Optimal Use of Enhanced Oil Recovery Polymers Under Hostile Conditions. PhD Thesis. The University of Texas at Austin. USA.
Levitt, D. B., & Pope, G. A. (2008). Selection and Screening of Polymers for Enhanced-Oil Recovery. (SPE 113845). SPE Improved Oil Recovery Symposium Held in Tulsa, Oklahoma, USA. DOI: 10.2118/113845-MS
Levitt, D. B., Pope, G. A., & Jouenne, S. (2010). Chemical Degradation of Polyacrylamide Polymers Under Alkaline Conditions. (SPE 129879). SPE Improved Oil Recovery Symposium Held in Tulsa, Oklahoma, USA.
Liu, P., Mu, Z., Wang, C., & Wang, W. (2017). Experimental Study of Rheological Properties and Oil Displacement Efficiency in Oilfields for a Synthetic Hydrophobically Modified Polymer. Sci. Rep., 7, 8791.
Luo, J. H., & Cheng, G.Y. (1993). Synthesis of Salinity-Resistant and High-Temperature- Resistant RTS Series of Polymers and their Properties. Oilfield Chemistry, 10 (4), 331–335.
Luo, J. H., Bu, R. Y., Wang, P. M., Bai, F. L., Zhang, Y., Yang, J. B., & Liu, Y. Z. (2002). Properties of KYPAM, a Salinity-Resistant Polymer Used in EOR. Oilfield Chemistry, 19 (1), 64–67.
Manrique, E. J., Muci, V. E., & Gurfinkel, M. E. (2007). EOR Field Experiences in Carbonate Reservoirs in the United States. SPEREE, 667–686.
Martin, C. A. G., & Páez, E. G. M. (2017). Efeito da salinidade na tensão interfacial do sistema óleo/ agua em condições isobáricas e incremento gradual da temperatura. Fuentes: El reventón energético, 15(2), 117-124.
McPhee, C., Reed J., & Zubizarreta, I. (2015). Core Analysis: Best Practices. First Edition, Amsterdam, Netherlands: Elsevier B. V.
Molano, A. M. J., Navarro, S. F. M., & Díaz, R. J. (2014). Metodología para el diseño de baches en un proceso de inyección de polímeros para recobro mejorado, considerando fenómenos de interacción roca/fluidos. Fuentes: El reventón energético, 12(2), 6.
Moradi-Araghi, A., & Doe, P. H. (1987). Hydrolysis and Precipitation of Polyacrylamide in Hard Brines at Elevated Temperatures. SPE Reservoir Engineering, 2, 189-198.
Morgan, S. E., & McCormick, C. L. (1990). Water- Soluble Polymers in Enhanced Oil Recovery. Progr. Polym. Sci., 15, 103-145.
Needham, R. B., & Doe, P. H. (1987). Polymer Flooding Review. JPT, 39 (12), 1503–1507.
Quadri, S. M. R., Shoaib, M., AlSumaiti, A. M., & Alhassan, S. M. (2015). Screening of Polymers for EOR in High Temperature, High Salinity and Carbonate Reservoir Conditions. (IPTC 18436). International Petroleum Technology Conference held in Doha, Qatar. DOI: 10.2523/IPTC-18436-MS
Parker Jr. W. O., & Lezzi, A. (1993). Hydrolysis of Sodium-2-Acrylamido-2- Methylpropanesulfonate Copolymers at Elevated Temperature in Aqueous Solution Via 13C.n.m.r. Spectroscopy. Polymer, 34, 4913-4918.
Pei, Y., Zhao, L., Du, G., Li, N., Xua, K., & Yang, H. (2016). Investigation of the Degradation and Stability of Acrylamide-Based Polymers in Acid Solution: Functional Monomer Modified Polyacrylamide. Petroleum, 2 (4), 399-407.
Pinto, M. S., Herrera, D. M., & Angarita, J. C. G. (2018). Production optimization for a conceptual model through combined use of polymer flooding and intelligent well technology under uncertainties. Revista Fuentes, 16(1), 37-45.
Pu, W-F., Liu, R., Peng, Q., Du, D-J., & Zhao, Q-N. (2016) Amphiphilically Modified Chitosan Copolymer for Enhanced Oil Recovery in Harsh Reservoir Condition. J. Ind. & Eng. Chemistry, 37, 216-223.
Raffa, P., Broekhuis, A. A., & Picchioni, F. (2016). Amphiphilic Copolymers Based on PEG‐ Acrylate as Surface Active Water Viscosifiers: Towards New Potential Systems for Enhanced Oil Recovery. J. Appl. Polym. Sci., 133 (42), 44100. DOI: 10.1002/app.44100
Rajapaksha, S., Britton, C., McNeil, R. I., Kim, D. H., Unomah, M., Kulawardana, E., Upamali, N., Weerasooriya, U. P., & Pope, G. A., (2014). Restoration of Reservoir Cores to Reservoir Condition before Chemical Flooding Tests. (SPE 169887). SPE Improved Oil Recovery Symposium, 12-16 April, Tulsa, Oklahoma, USA. DOI: 10.2118/169887-MS.
Rashidi, M., Blokhus, A. M., & Skauge, A. (2010). Viscosity Study of Salt Tolerant Polymers. J. Appl. Polym. Sci., 117 (3), 1551-1557.
Ryles, R. G. (1988). Chemical Stability Limits of Water-Soluble Polymer Used in Oil Recovery. SPERE, 3, 23-34.
Sabhapondit, A., Borthakur, A., & Haque, I. (2003). Characterization of Acrylamide Polymers for Enhanced Oil Recovery. J. Appl. Polym. Sci., 87 (12), 1869-1878.
Sarkar, N., & Kershner, L. D. (1996). Rigid Rod Water-Soluble Polymers. J. Appl. Polym. Sci., 62 (2), 393-408.
Scoggins, M. W., & Miller, J. W. (1979). Determination of Water-Soluble Polymers Containing Primary Amide Groups Using the Starch-Triiodide Method. SPEJ, 19 (3):151- 154.
Seright, R. S., Campbell, A. R., & Mozley, P S. (2010). Stability of Partially Hydrolyzed Polyacrylamides at Elevated Temperatures in the Absence of Divalent Cations. SPEJ., 14, 341-348.
Seright, R. S., & Henrici, B. J. (1990). Xanthan Stability at Elevated Temperatures. SPERE, 5, 51-60.
Seright, R. S., Seheult, J. M., & Talashek, T. (2008). Injectivity Characteristics of EOR Polymers. (SPE 115142). SPE Annual Technical Conference and Exhibition, Denver, Colorado, USA.
Seright, R. S., Fan, T., Wayrik, K. E., & Wan, H. (2011). Rheology of a New Sulfonic Associative Polymer in Porous Media. (SPE 141355). SPE International Symposium on Oilfield Chemistry held in The Woodlands, Texas, USA.
Sheng, J. J. (2011). Modern Chemical Enhanced Oil Recovery: Theory and Practice. Burlington, Massachusetts, USA: Gulf Professional, Elsevier Inc.
Shupe, R. D. (1981). Chemical Stability of Polyacrylamide Polymers. JPT, 8 (33), 1513-1530.
Sorbie, K. S. (1991). Polymer-Improved Oil Recovery. CRC Press: Boca Raton, FL.
Sukpisan, J., Kanatharana, J., Sirivat, A., & Wang, S. (1998). The Specific Viscosity of Partially Hydrolyzed Polyacrylamide Solutions: Effects of Degree of Hydrolysis, Molecular Weight, Solvent Quality and Temperature. J. Polym Sci B: Polym Phys., 36 (5), 743-753.
Tapias-Hernández, F. A., Lizcano, J. C., Zanoni- Lopes-Moreno, R. B. (2018). Effects of Salts and Temperature on Rheological and Viscoelastic Behavior of Low Molecular Weight HPAM Solutions. Revista Fuentes: El Reventón Energético, 16 (1), 19-35.
Terayama, H. (1952). Method of Colloid Titration (A New Titration Between Polymer Ions). Journal of Polymer, 8 (2), 243-253.
Yalcin, T., Dai, Y., & Li, L. (1998). Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for Polymer Analysis: Solvent Effect in Sample Preparation. Journal of the American Society for Mass Spectrometry, 9 (12), 1303-1310.
Yerramilli, S. S., Zitha, P. L., & Yerramilli, R. C. (2013). Novel Insight into Polymer Injectivity for Polymer Flooding. SPE European Formation Damage Conference and Exhibition held in Noordwijk, The Netherlands.
You, Q., Han, F. L., Wang, Y. F., & Mu, L. N. (2007). Comparison of the Properties of Injected and Released Polyacrylamide in Polymer Flooding. Journal of Beijing University of Chemical Technology, 34, 414-417.
Wolf, B. A. (1985). Solubility of Polymers. Pure & Appl. Chem., 57 (2), 323-336.
Wu, Y., Mahmoudkhani, A, Watson, P., Fenderson, T. R., & Nair, M. (2012). Development of New Polymers with Better Performance under Conditions of High Temperature and High Salinity. SPE EOR Conference at Oil and Gas West Asia held in Muscat, Oman.
Zurimendi, J. A., Guerrero, S. J., & Leon, V. (1984). The Determination of the Degree of Hydrolysis in Poly(acrylamides): Simple Methods using C13 NMR and Elementary Analysis. Polymer, 25 (9), 1314-1316.