Astaxanthin production by Haematococcus pluvialis under the phosphate deficiency effect and hight light intensity
Published 2024-11-20
Keywords
- Microalgae,
- Carotenoid,
- Phosphate deficiency,
- Grow medium,
- Hight light intensity
How to Cite
Copyright (c) 2024 Judith Elena Camacho Kurmen, Natalia Rodriguez Rodriguez

This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Abstract
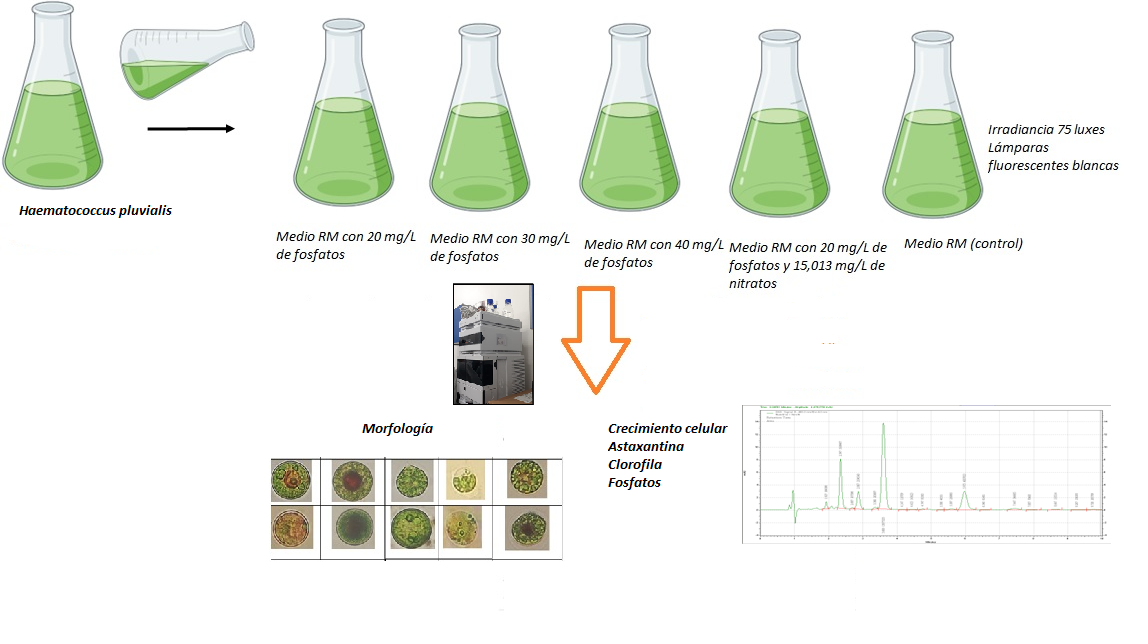
Astaxanthin is a carotenoid produced by the microalgae Haematococcus pluvialis, which can accumulate up to 3 % of Astaxanthin in dry weight. It is produced under stress conditions, such as increased light and nutrient limitation. The objective is to establish conditions for H. pluvialis to produce astaxanthin using phosphate deficiency in the Rudic medium (RM).
Bioreactors with H. pluvialis UTEX 2505 were used, which contained 20 mg/L (RM1), 30 mg/L (RM2), 40 mg/L (RM3) phosphate, 20 mg/L phosphate with 15,013 mg/L of nitrate (RM4) and a control (RM5). A pH of 6.8, photoperiod 20 h light and 4h darkness and irradiance of 75 luxes in stress phase, white light, temperature of 20 ± 1 °C was used. The fastest growing treatment was RM3 with 9.69 x 105 cell/mL; The ANOVA (95 %) didn’t establish significant differences between treatments (F: 0.272; P: 0.895; df: 4). The highest concentration of chlorophyll was by RM3, with 26.2 µg/ mL (27 pg/cell); no significant differences were established between treatments (F: 1.053; P: 0.392; df: 4). In addition, RM3 obtained higher phosphate consumption (62.5 %); with significant differences between treatments (F: 3.887; P: 0.011; df: 4). The morphological changes demonstrated an accumulation of aplanospores cells for RM1 and RM2 treatments. The RM2 treatment obtained higher astaxanthin with 5,772 µg/mL (6.11 pg/cell); no significant differences were established between treatments (F: 0.622; P: 0.649; df: 4). It was established that phosphate deficiency combined with high light intensity, increases the production of astaxanthin in H. pluvialis.
Downloads
References
- Richmond A. Biological principles of mass cultivation of photoautotrophic microalgae. En: Handbook of microalgal culture: applied phycology and biotechnology. 2 ed. Richmond A, Hu Q, editor. Wiley-Blackwell; 2013. p. 169-204. https://doi.org/10.1002/9781118567166.ch11
- García MC, Acién FG, Del Río E, Fernández JM, Cerón CM, Guerrero M, et al. Production of Astaxanthin by Haematococcus pluvialis: Taking the One-Step System Outdoors. Biotechnol. Bioeng. 2009;102:651-657. https://doi.org/10.1002/bit.22076
- Li J, Zhu D, Niu J, Shen S, Wang G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnology Advances. 2011;29(6):568-574. https://doi.org/10.1016/j.biotechadv.2011.04.001
- Shah MR, Liang Y, Cheng YJ, Daroch M. Astaxanthin- Production Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016;7(531):1-28. https://doi.org/10.3389/fpls.2016.00531
- Mussagy CU, Kot A, Dufossé L, Gonçalves CNDP, Pereira JFB, Santos-Ebinuma VC, et al. Microbial astaxanthin: from bioprocessing to the market recognition. Applied Microbiology and Biotechnology, 2023;107:4199-4215. https://doi.org/10.1007/s00253-023-12586-1
- Camacho JE, González G, Klotz B. Producción de astaxantina en Haematococcus pluvialis bajo diferentes condiciones de estrés. NOVA. 2013;11(19):93-104.
- Córdoba Astroc N, Acero Reyes N, Duque Buitrago L, Jiménez Aguilar J, Serna Jiménez JA. Obtención y caracterización de astaxantina de la microalga Haematococcus pluvialis. UGCiencia. 2015;21:73-82. https://doi.org/10.18634/ugcj.21v.1i.426
- Niño Castillo CM, Rodríguez Rivera FC, Díaz LE, Lancheros Díaz AG. Evaluación de las condiciones de crecimiento celular para la producción de astaxantina a partir de la microalga Haematococcus pluvialis. NOVA. 2017;15(28):19-31.
- He P, Ducan J, Barber J. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis: Effects of Cultivation Parameters. Journal of Integrative Plant Biology. 2007;49(4):447-451. https://doi.org/10.1111/j.1744-7909.2007.00468.x
- López GL, Ponce T, Flores CM, Cristini E, Cañizares RO. Estimulación de la carotenogénesis en Haematococcus pluvialis mediante la adición de CO2 . En: XIII Congreso Nacional de Biotecnología y Bioingeniería. 2009.
- Imamoglu E, Conk M, Vardar F. Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotechnology. 2009;26(3-4):199-204. https://doi.org/10.1016/j.nbt.2009.08.007
- Kang CD, Han SJ, Choi SP, Sim SJ. Fed-batch culture of astaxanthin-rich Haematococcus pluvialis by exponential nutrient feeding and stepwise light supplementation. Bioprocess Biosyst Eng. 2010;33:133-139. https://doi.org/10.1007/s00449-009-0362-5
- Nagaraja S, Arulmurugana P, Rajarama MG, Sundararajb R, Rengasamy R. Enhanced production of astaxanthin at different physico-chemical parameters in the green alga Haematococcus pluvialis Flotow. Phykos. 2012;42(1):59– 71.
- Xu Jiajie XJ, Galan A, Zhao Fengmin ZF, Cao Youfu CYF, Liu Wei LW, Su Xiurong SX. Kinetic model analysis of Haematococcus pluvialis in a cultivation optimization to accumulate lipids. IAEJ. 2012;22(3-4):74-81.
- Chul J, Phill C, Hong ME, Jun S. Enhanced astaxanthin production from microalga Haematococcus pluvialis by two- stage perfusion culture with stepwise light irradation. Bioprocess Biocyst Eng. 2014;37:2039-2047. https://doi.org/10.1007/s00449-014-1180-y
- Kumar C. Studies on Production Potential of Astaxanthin by Haematococcus pluvialis (Tesis doctoral). Nueva Delhi, India: Indian Agricultural Research Institute; 2014.
- Miranda A, Meneses O, Hoyos H, Sáez A, Vargas G. Evaluación del efecto de las bajas concentraciones de fosfato en el crecimiento y acumulación de astaxantina en Haematococcus pluvialis UTEX 2505. III Congreso Colombiano de Bioquímica y Biología Molecular. 2018.
- Miranda AM, Ossa EA, Vargas GJ, Sáez AA. Efecto de las bajas concentraciones de Nitratos y Fosfatos sobre la Acumulación de Astaxantina en Haematococcus pluvialis UTEX 2505. Inf. tecnol. 2019;30(1):23-32. http://dx.doi.org/10.4067/S0718-07642019000100023
- Li F, Cai M, Lin M, Huang X, Wang J, Zheng X, et al. Accumulation of Astaxanthin Was Improved by the Nonmotile Cells of Haematococcus pluvialis. BioMed Research International. 2019;2019:8101762. https://doi.org/10.1155/2019/8101762
- Gómez L, Orozco MI, Quiroga C, Díaz JC, Huérfano J, Díaz LE, et al. Producción de Astaxantina y expresión de genes en Haematococcus pluvialis (Chlorophyceae, Volvocales) bajo condiciones de estrés por deficiencia de nitrógeno y alta irradiancia. Revista Mutis. 2019;9(2):7-24. https://doi.org/10.21789/22561498.1532
- Galvão RM, Sanatana TS, Fontes CH, Sales EA. Modeling of Biomass Production of Haematococcus pluvialis. Applied Mathematics. 2013;4:50-56. https://doi.org/10.4236/am.2013.48A008
- Imamoglu E, Vardar Sukan F, Conk Dalay M. Effect of different culture media and light intensities on growth of Haematococcus pluvialis. Int. J. Nat. Sci. 2007;1(3):05-09.
- Sun H, Guan B, Kong Q, Geng Z, Wang. Repeated cultivation: noncell disruption extraction of astaxanthin for Haematococcus pluvialis. Scientific Reports. 2016;6(20578):1-12. https://doi.org/10.1038/srep20578
- Ding D, Chen S, Peng S, Jiang C, Zheng L, Li J. Strategies of phosphorus utilization in an astaxanthin producing green alga Haematococcus pluvialis, a comparison with a bloom-forming cyanobacterium Microcystis wesenbergii. Aquat Ecol. 2019;53:679-688. https://doi.org/10.1007/s10452-019-09718-z
- Yuan J-P, Chen F. Hydrolysis Kinetics of Astaxanthin Esters and Stability of Astaxanthin of Haematococcus pluvialis during Saponification. J. Agric. Food Chem. 1999;47:31-35. https://doi.org/10.1021/jf980465x
- Tocquin P, Fratamico A, Franck F. Screening for a low-cost Haematococcus pluvialis medium reveals an unexpected impact of a low N/P ratio on vegetative growth. J Appl Phycol. 2012;24,365–373. https://doi.org/10.1007/s10811-011-9771-3
- Baranyi J, Roberts T. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994;23(3):277-294.https://doi.org/10.1016/0168-1605(94)90157-0
- Wang J, Sommerfeld MR, Lu C, Hu Q. Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae. 2013;28(2):193-202. https://doi.org/10.4490/algae.2013.28.2.193
- Liyanaarachchi VC, Nishshanka GKSH, Premaratne RGMM, Ariyadasa TU, Nimarshana PHV, Malik A. Astaxanthin accumulation in the green microalga Haematococcus pluvialis: Effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnology Reports, 2020;28:e00538. https://doi.org/10.1016/j.btre.2020.e00538
- Liu JG, van der Meer J, Zhang LT, Zhang Y. Cultivation of Haematococcus pluvialis for astaxanthin production. En: Microalgal Production for Biomass and High-Value Products. Slocombe SP, Benemann JR, editor. New York, USA: CRC Press; 2016. p. 267–293.
- Ranjbar R, Inoue R, Shiraishi H, Katsuda T, Katoh S. High efficiency production ofastaxanthin by autotrophic cultivation of Haematococcus pluvialis in a bubble column photobioreactor. Biochem. Eng. J. 2007;39(3):575-580. https://doi.org/10.1016/j.bej.2007.11.010

