Electrodos de óxido de grafeno modificados con peroxidasa de cáscara de batata para la detección de peróxido de hidrógeno mediante sensado electroquímico
Publicado 2024-12-09
Palabras clave
- Cáscara de batata,
- Peroxidasa,
- Voltamperometría cíclica,
- Detección,
- Peróxido de hidrógeno
Cómo citar
Derechos de autor 2024 John J. Castillo, Miguel Ángel Vega

Esta obra está bajo una licencia internacional Creative Commons Atribución-SinDerivadas 4.0.
Resumen
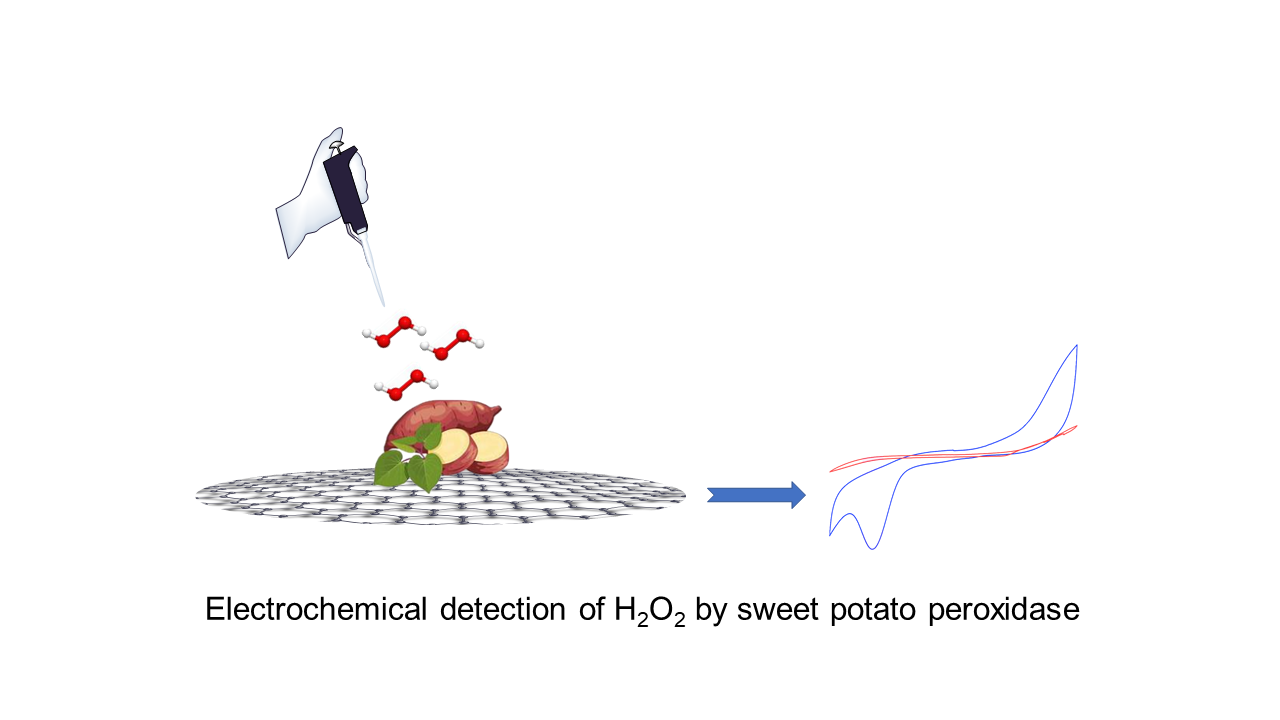
El desarrollo de métodos de detección eficientes y sensibles para el peróxido de hidrógeno (H2O2) es crucial para diversas aplicaciones en biología, medicina y monitoreo ambiental. Aquí, presentamos un enfoque novedoso que utiliza electrodos de óxido de grafeno (OG) impresos en pantalla modificados con extracto de peroxidasa de cáscara de batata (PCB) para la detección electroquímica de H2O2. La BPP se caracterizó por tener una actividad específica de 478 Umg-1, un pH óptimo de 8.0 y una termoestabilidad a 60°C con un Kinact de 7.02x10-3min-1. En este estudio, investigamos sistemáticamente el proceso de fabricación de los electrodos modificados con PCB y caracterizamos su rendimiento electroquímico utilizando la técnica de voltamperometría cíclica (VC). El PCB-ESOG demuestra un rendimiento electrocatalítico sobresaliente para la reducción de H2O2, mostrando una respuesta lineal en el rango de concentración de 250 μM a 5 mM y un límite de detección de 4.6 mM. Este novedoso sensor, creado mediante la incorporación de BPP en el electrodo de OG, ofrece un sistema de detección electroquímica prometedor para medir H2O2 en muestras reales, lo cual tiene importantes aplicaciones biomédicas y ambientales. En general, este estudio presenta una estrategia versátil y eficiente para la detección electroquímica de H2O2 utilizando BPP-ESOG, explorando el camino para metodologías analíticas avanzadas con amplias aplicaciones en biología.
Descargas
Referencias
- Caglar B, İçer F, Özdokur KV, Caglar S, Özdemir AO, Guner EK, et al. A novel amperometric H2O2 biosensor constructed by cress peroxidase entrapped on BiFeO3 nanoparticles. Mater Chem Phys. 2021;262:124287. https://doi.org/10.1016/j.matchemphys.2021.124287
- T.sriwong K, Matsuda T. Recent Advances in Enzyme Immobilization Utilizing Nanotechnology for Biocatalysis. Org. Process Res. Dev. 2022;26(7):1857–1877. https://doi.org/10.1021/acs.oprd.1c00404
- Bollella P. Enzyme-based amperometric biosensors: 60 years later … Quo Vadis?. Analytica Chimica Acta. Elsevier B.V.; 2022;1234:340517. https://doi.org/10.1016/j.aca.2022.340517
- Tseng CH, Lin HH, Hung CW, Cheng IC, Luo SC, Cheng IC, et al. Electropolymerized Poly(3,4-ethylenedioxythiophene)/Screen-Printed Reduced Graphene Oxide-Chitosan Bilayer Electrodes for Flexible Supercapacitors. ACS Omega 2021;6(25):16455–16464. https://doi.org/10.1021/acsomega.1c01601
- Gross MA, Paterno LG. Iron Oxide, Reduced Graphene Oxide, and Electrodeposited Gold Nanoparticle-Based Electrodes for Nanomolar Detection of Nitrite in Food. ACS Appl. Nano Mater. 2024;7(8):9542–9553. https://doi.org/10.1021/acsanm.4c01016
- Castillo J, Guarin-Guio PA, Ortiz L. Bio-Electrocatalytic Reduction of Hydrogen Peroxide by Peroxidase from Guinea Grass (Panicum Maximum) Immobilized on Graphene and Graphene Oxide Screen-Printed Electrodes. Ing. Univ. 2022;26. https://doi.org/10.11144/javeriana.iued26.brhp
- Valsalakumar VC, Joseph AS, Piyus J, Vasudevan S. Polyaniline-Graphene Oxide Composites Decorated with ZrO2 Nanoparticles for Use in Screen-Printed Electrodes for Real-Time l-Tyrosine Sensing. ACS Appl. Nano Mater. 2023;6(10):8382–8395. https://doi.org/10.1021/acsanm.3c00659
- Mathé C, Barre A, Jourda C, Dunand C. Evolution and expression of class III peroxidases. Arch Biochem Biophys. 2010;500(1):58–65. https://doi.org/10.1016/j.abb.2010.04.007
- de Oliveira FK, Santos LO, Buffon JG. Mechanism of action, sources, and application of peroxidases. Food Research International. 2021;143:110266 https://doi.org/10.1016/j.foodres.2021.110266
- Hiner ANP, Ruiz JH, Rodri JN, López A, Cánovas FG, Brisset NC, et al. Reactions of the class II peroxidases, lignin peroxidase and Arthromyces ramosus peroxidase, with hydrogen peroxide: Catalase-like activity, compound III formation, and enzyme inactivation. J Bio Chem. 26;277(30):26879–85. https://doi.org/10.1074/jbc.M200002200
- Freitas CDT, Costa JH, Germano TA, de O. Rocha R, Ramos M V., Bezerra LP. Class III plant peroxidases: From classification to physiological functions. International Journal of Biological Macromolecules. 2024;263:130306. https://doi.org/10.1016/j.ijbiomac.2024.130306
- Kotchey GP, Zhao Y, Kagan VE, Star A. Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo. Adv Drug Deliv Rev. 2013;65(15):1921–32. https://doi.org/10.1016/j.addr.2013.07.007
- Li G, Miao P. Electrochemical Analysis of Proteins and Cells [Internet]. Berlin, Germany: Springer Berlin, Heidelberg; 2012. https://doi.org/10.1007/978-3-642-34252-3
- Vatankhahan H, Esteki F, Jabalameli MA, Kiani P, Ehtiati S, Movahedpour A, et al. Electrochemical biosensors for early diagnosis of glioblastoma. Clinica Chimica Acta. 2024;557:117878. https://doi.org/10.1016/j.cca.2024.117878
- Škulj S, Kožić M, Barišić A, Vega A, Biarnés X, Piantanida I, et al. Comparison of two peroxidases with high potential for biotechnology applications – HRP vs. APEX2. Comput Struct Biotechnol J. 2024;23:742–51. https://doi.org/10.1016/j.csbj.2024.01.001
- Villamizar EN, Ríos CA, Castillo JJ. A Hydrogen Peroxide Biosensor Based on the Immobilization of the Highly Stable Royal Palm Tree Peroxidase (Roystonea regia) with Chitosan and Glutaraldehyde on Screen-printed Graphene Electrodes. Chem. Soc. 2016;60(3):135-140. https://doi.org/10.29356/jmcs.v60i3.95
- Sakharov IY, Vesgac B MK, Galaev IY, Sakharova IV, Pletjushkina OY. Peroxidase from leaves of royal palm tree Roystonea regia: Purification and some properties. Plant Science. 2001;161(5):853–60. https://doi.org/10.1016/S0168-9452(01)00466-6
- Watanabe L, de Moura PR, Bleicher L, Nascimento AS, Zamorano LS, Calvete JJ, et al. Crystal structure and statistical coupling analysis of highly glycosylated peroxidase from royal palm tree (Roystonea regia). J Struct Biol. 2010;169(2):226–42. https://doi.org/10.1016/j.jsb.2009.10.009
- Jiménez-Villalba K, Arrieta-Banquet L, Salcedo-Mendoza J, Contreras-Lozano K. Characterization of batatas flours and starches (Ipomoea batatas Lam.) from the colombian caribbean coast. Revista UDCA Actualidad and Divulgacion Cientifica. 2019;22(1):1-11. https://doi.org/10.31910/rudca.v22.n1.2019.1185
- Lago Castro L. El cultivo de la batata: una oportunidad agroalimentaria para pequeños productores de clima cálido. Colombia: SENA, SAC; 2011. Available from: http://hdl.handle.net/20.500.12324/13373
- Renee Vidal A, Linaloe Zaucedo-Zuñiga A, de Lorena Ramos-García M. Propiedades nutrimentales del camote (Ipomoea batatas L.) y sus beneficios en la salud humana. Revista Iberoamericana de Tecnología Postcosecha: 2018;19(2).
- Centeno DA, Solano XH, Castillo JJ. A new peroxidase from leaves of guinea grass (Panicum maximum): A potential biocatalyst to build amperometric biosensors. Bioelectrochemistry. 2017;116:33–8. https://doi.org/10.1016/j.bioelechem.2017.03.005
- Dequaire M, Limoges B, Moiroux J, Savéant JM. Mediated electrochemistry of horseradish peroxidase. Catalysis and inhibition. J Am Chem Soc. 2002;124(2):240–53. https://doi.org/10.1021/ja0170706
- Alpeeva IS, Niculescu-Nistor M, Leon JC, Csöregi E, Sakharov IY. Palm tree peroxidase-based biosensor with unique characteristics for hydrogen peroxide monitoring. Biosens Bioelectron. 2005;21(5):742–8. https://doi.org/10.1016/j.bios.2005.01.008
- Saud Al-Bagmi M, Shahnawaz Khan M, Alhasan Ismael M, Al-Senaidy AM, Ben Bacha A, Mabood Husain F, et al. An efficient methodology for the purification of date palm peroxidase: Stability comparison with horseradish peroxidase (HRP). Saudi J Biol Sci. 2018;26(2):301–7. https://doi.org/10.1016/j.sjbs.2018.04.002
- Yuan M, Zhao H, Huang Q, Liu X, Zhou Y, Diao X, et al. Comparison of three palm tree peroxidases expressed by Escherichia coli: Uniqueness of African oil palm peroxidase. Protein Expr Purif. 2021;179:105806. https://doi.org/10.1016/j.pep.2020.105806
- Mirzaei MS, Ivanov M V., Taherpour AA, Mirzaei S. Mechanism-Based Inactivation of Cytochrome P450 Enzymes: Computational Insights. Chem. Res. Toxicol. 2021:34(4):959–87. https://doi.org/10.1021/acs.chemrestox.0c00483
- Seelajaroen H, Bakandritsos A, Otyepka M, Zbořil R, Sariciftci NS. Immobilized Enzymes on Graphene as Nanobiocatalyst. ACS Appl Mater Interfaces. 2020;12(1):250–9. https://doi.org/10.1021/acsami.9b17777
- Ferapontova EE, Castillo J, Hushpulian D, Tishkov V, Chubar T, Gazaryan I, et al. Direct electrochemistry of recombinant tobacco peroxidase on gold. Electrochem commun. 2005;7(12):1291–7. https://doi.org/10.1016/j.elecom.2005.09.004
- Saud Al-Bagmi M, Shahnawaz Khan M, Alhasan Ismael M, Al-Senaidy AM, Ben Bacha A, Mabood Husain F, et al. An efficient methodology for the purification of date palm peroxidase: Stability comparison with horseradish peroxidase (HRP). Saudi J Biol Sci [Internet]. 2019;26(2):301–7. https://doi.org/10.1016/j.sjbs.2018.04.002
- Gaspar S, Popescu IC, Gazaryan IG, Bautista AG, Sakharov IY, Mattiasson B, et al. Biosensors based on novel plant peroxidases: a comparative study. Electrochimica Acta. 2000;46(2-3):255-264. https://doi.org/10.1016/S0013-4686(00)00580-6
- Zhang X, Lou J, Yuan J, Xu J, Fan X. Style decolorization treatment of denim fabric: Decomposition of indigo dyes via horseradish peroxidase/H2O2 system at room temperature. Sustain Chem Pharm. 2023;35:101233. https://doi.org/10.1016/j.scp.2023.101233
- Castillo J, Gáspár S, Sakharov I, Csöregi E. Bienzyme biosensors for glucose, ethanol and putrescine built on oxidase and sweet potato peroxidase. Biosensors and Bioelectronics. 2003;18(5-6):705–14. https://doi.org/10.1016/S0956-5663(03)00011-3
- Adachi T, Kitazumi Y, Shirai O, Kano K. Direct electron transfer-type bioelectrocatalysis of redox enzymes at nanostructured electrodes. Catalysts. 2020;10(2):236. https://doi.org/10.3390/catal10020236
- Bhapkar S, Choudhari U, Jadhav U, Jagtap S. Evaluation of soybean peroxidase - Copper phosphate mediated organic-inorganic hybrid for hydrogen peroxide biosensor application. Sensors International. 2023;4:100242. https://doi.org/10.1016/j.sintl.2023.100242
- Orduz AE, Gutiérrez JA, Blanco SI, Castillo JJ. Amperometric detection of triclosan with screen-printed carbon nanotube electrodes modified with Guinea Grass (Panicum maximum) peroxidase. Universitas Scientiarum. 2019;24(2):363–79. https://doi.org/10.11144/Javeriana.SC24-2.adot
- Guarín P, Cano HJ, Castillo JJ. Detección electroquímica de peróxido de hidrógeno usando peroxidasa de pasto Guinea (Panicum maximum) inmovilizada sobre electrodes serigrafiados de puntos cuánticos. rev.ion. 2019;32(2):67-76. https://doi.org/10.18273/revion.v32n2-2019007
- Lai GS, Zhang HL, Han DY. Amperometric hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase by carbon-coated iron nanoparticles in combination with chitosan and cross-linking of glutaraldehyde. Microchimica Acta. 2009 Apr;165(1–2):159–65.
- Guarín P, Cristancho J, Castillo JJ. Rapid electrochemical detection of Staphylococcus aureus. Available from: https://doi.org/10.18257/raccefyn.1019

